Applications
Cross-species Vaccines
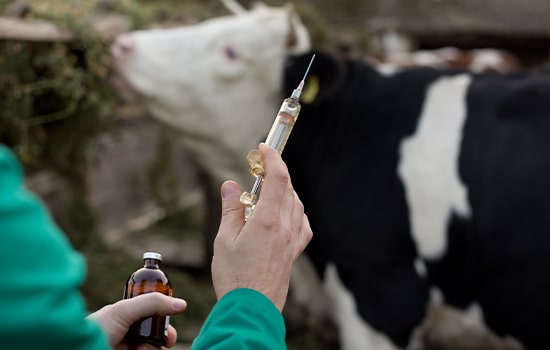
Also within the context of One Health approach, the separation of funding for studies of vaccine for humans and animals further subdivide the available protective technologies which could be system-transcending. In the study conducted by Warimwe et. al, adenovirus platform was used to develop vaccines for animals and humans. It is already known that adenoviruses can be utilized for human gene therapy applications. It was demonstrated in the study that conjugating Rift Valley Fever virus antigen to a chimpanzee respiratory adenovirus provided 100% protection on multiple livestock after administration. The developed Rift Valley Fever vaccine is planned to be deployed for human clinical trials.
References:
- Basinski, A. J., Nuismer, S. L., & Remien, C. H. (2019). A little goes a long way: Weak vaccine transmission facilitates oral vaccination campaigns against zoonotic pathogens. Plos Neglected Tropical Diseases, 13(3), e0007251.
- Center for Disease Control and Prevention (2017). Prioritizing and Preventing Deadly Zoonotic Diseases. Last accessed 26 August 2019 from https://www.cdc.gov/globalhealth/healthprotection/fieldupdates/winter-2017/prevent-zoonotic-diseases.html
- Peninah M. Munyua, M. Kariuki Njenga, Eric M. Osoro, Clayton O. Onyango, Austine O. Bitek, Athman Mwatondo, … Marc-Alain Widdowson. (2019). Successes and challenges of the One Health approach in Kenya over the last decade. BMC Public Health, (S3), 1. https://doi.org/10.1186/s12889-019-6772-7
- The Conversation (2019). We’re developing the world’s first vaccine suitable for humans and livestock. Last accessed 19 June 2019 from https://theconversation.com/were-developing-the-worlds-first-vaccine-suitable-for-humans-and-livestock-115406





