Applications
Companion Animal mAbs
Reducing pain thru monoclonal antibodies
The available options for pain management for cats and dogs include small molecule drugs such as narcotics, non-steroidal anti-inflammatory drugs (NSAIDs), and polysulphated glycosaminoglycans (PSGAG). These drugs are still effective for relieving pain yet account for many animal drug side effect reports to veterinary centers worldwide. NSAIDs interfere with the natural function of prostaglandins, which aside from promoting inflammatory effects, also support platelet clotting and protect the lining of the stomach from acid. These drugs are meant to be used according to the label. Furthermore, cats are more sensitive to the side effects of NSAIDs and no NSAIDs are approved, to date, by the US FDA for long-term use in cats. The side effects of these drugs would be a concern when administering against chronic pain.
Preliminary studies of anti-NGF were conducted and proven efficacious in rats as animal models. It is already common and well-understood technique for rodent to human conversion of antibodies. The technique commonly used is complementary-determining region (CDR) grafting or antibody reshaping. In this method, the rat antibody CDR region responsible for binding to the target epitope is transferred to a human IgG replacing the original human CDR region. The resulting antibody is a human antibody with rat chimera; retaining the specificity to the target while reducing the human anti-rat antibody response. Several modifications to the technique require balancing of anti-rat response and affinity of the antibody. The resulting anti-human NGF mAbs have already shown promising results in clinical trials.
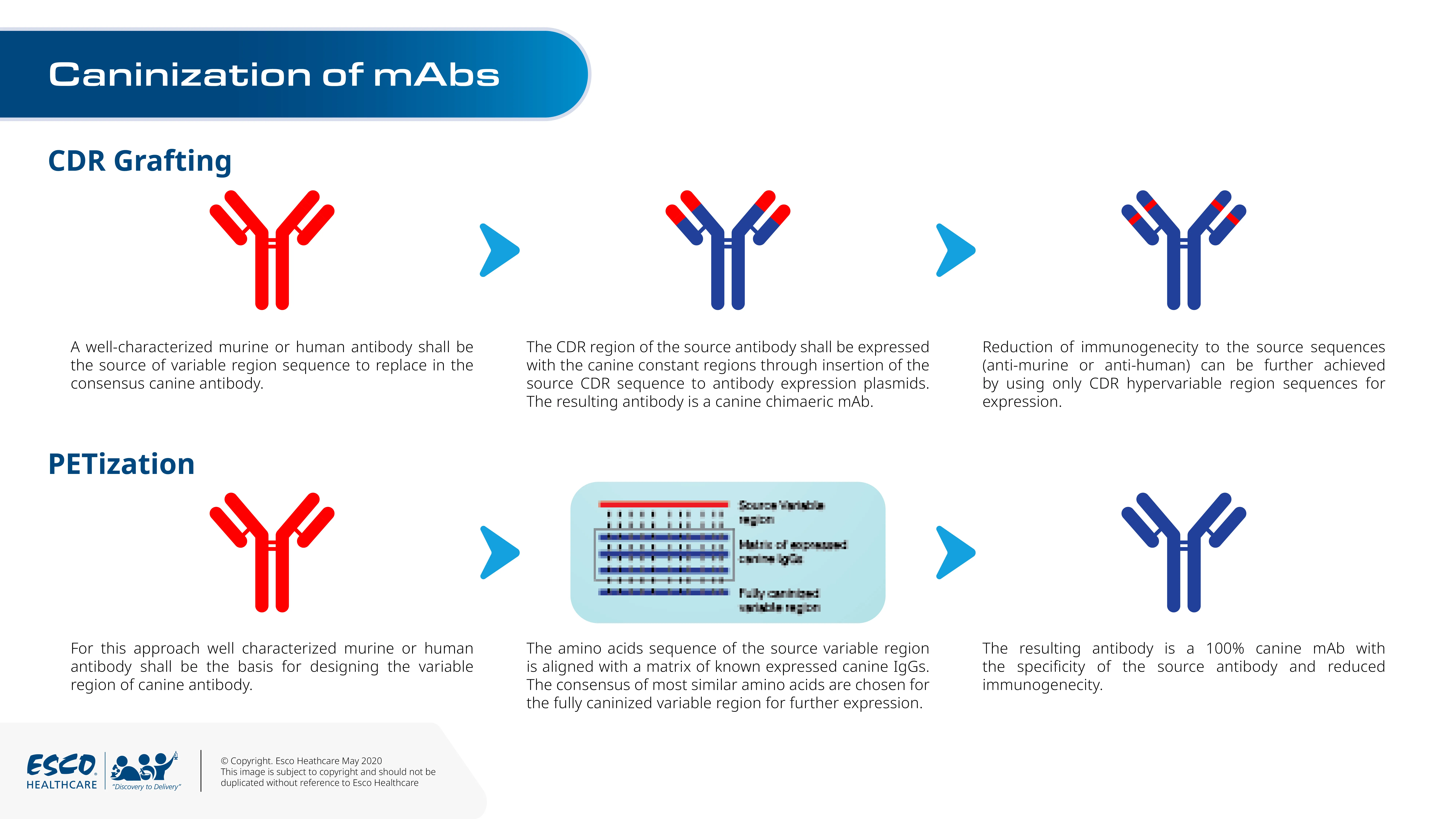
NGF and its receptors are highly conserved between dogs and humans. This allows the possibility to develop caninised version of the rat anti-NGF antibodies. David Gearing and colleagues at the Monash University in Australia developed a novel approach, termed PETization, to design canine from rat antibodies. Instead of using germline and nucleotide sequence for the framework of the antibody, the novel approach uses a matrix of expressed canine IgG sequences.
In the study, the designed anti-NGF canine mAbs were expressed in CHO cells in a fed-batch culture system. The supernatant contained the mAbs that were further purified using ion-exchange chromatography. Using the kaolin model, animals that were injected once with the anti-NGF canine mAbs matched the lameness reduction of animals given a daily dose of a commercial small molecule drug.
The single-dose prolonged effect makes antibody therapy more suitable for chronic pain management such as cancer, osteoarthritis, and post-surgery in animals.
Cancer therapy using monoclonal antibodies
Canine lymphoma is similar to human non-Hodgkin’s lymphoma in terms of pathology and response to therapy; thus, the effectiveness of immunotherapy in humans can also be translated to dogs. CD20 and CD52-specific mAbs have been approved by the US Department of Agriculture for B cell and T cell lymphoma, respectively. The cross-species reactivity found in human-mice mAbs is being applied and tested in canines for different biologics such as bevacizumab and rituximab.
There is limited information on canine antigens expressed in B and T cells due to the lack of basic research in cancer tumor phenotyping. Funding on canine cancer research would help in the development of canine-specific reagents to help advance canine cancer tumor knowledge.
Diagnosis using monoclonal antibodies
The specificity feature of monoclonal antibodies can also be applied to detect antigens associated with diseases. Parasitic antigens, including Dirofilaria and Trichinella, found in the sera of dogs and cats can be detected using specific mAbs in various ELISA platforms (i.e, sandwich ELISA). Avian paramyxovirus type 1 in pet birds such as pigeons have also been diagnosed using mAbs.
Diagnostic marker research has identified several important markers that can be used to design specific mAbs. As an example, Serum amyloid A is a diagnostic marker which is elevated during an inflammatory response. The marker can be used to determine the response of the animal to therapy.
Combined with flow cytometry, mAbs can also be used for phenotyping companion animal cancer cells. This is essential to provide the accurate and best treatment available for the companion animal.
Production of biologics such as monoclonal antibodies and vaccines for therapeutic and diagnostic applications can be performed efficiently through our bioreactors. Our TideXcell™ bioreactors provide low shear, foam or bubbling-free, and high aeration culture environment with improved cell growth, thereby increasing the secreted yield of biological products.





