Applications
Autologous Cell Therapy
Autologous cell therapy is composed of various technologies and disciplines ranging from cellular and molecular biology to virology. The use of autologous cell therapy has been reported from as early as 1985 where autologous conjunctival transplants were used for the restoration of damaged ocular surfaces. Currently, this therapy has broad applications in modern medicine and plastic surgery ranging from the treatment of various ailments, improvement of wound healing, to life saving operations, and even cosmetic surgery.
Autologous cell therapy overview
Autologous Cell Therapy (ACT) is a novel therapeutic approach that uses an individual’s cells, which are cultured, engineered, and expanded ex vivo, and introduced back to the patient. This approach has achieved positive feedback for it minimizes risks of immunological adverse reactions, bio- incompatibility, and transmission-associated diseases (e.g. GVHD).
The scope for autologous cell therapy is vast and has immense potential for rejuvenation. Much of this form of therapy is currently under investigation or in clinical trials. These include the treatment of limb ischemia, bone marrow mononuclear cell transplantation treatment for acute radiation victims, ischemic stroke, and ischemic heart disease, cell-mediated immunotherapy after chemotherapy, autologous hematopoietic cells as targets for gene transfer to treat various blood disorders, and autoimmune diseases. Other applications are use of autologous macrophages to treat acute complete spinal cord injury, cell therapy with autologous lymphocytes to treat various cancers, cell therapy for inner ear cell degeneration, and autologous serum for treatment of ocular disorders.
Blood-forming stem cell transplants
An autologous stem cell transplant replaces a patient’s stem cells that were destroyed by treatment with radiation or high doses of chemotherapy. An autologous stem cell transplant is most often used to treat blood cancers, such as leukemia and lymphoma.
Autologous stem cell transplant includes a few steps. The first step is taking blood from a vein in the patient’s arm and flowing through an apheresis machine that removes the stem cells. The remaining blood is returned to a vein in the other arm. After that, the patient receives chemotherapy to kill cancer cells. Healthy cells, including blood-forming cells, are also destroyed by cancer treatment. The third step is when the patient completes chemotherapy or radiation therapy (not shown), the stem cells are given back to the patient to replace the blood-forming cells that were destroyed by the cancer treatment. The stem cells help the bone marrow recover and make healthy blood cells.
CAR-T Cell Therapy
For decades, the foundations of cancer treatment have been surgery, chemotherapy, and radiation therapy. But new categories of treatment have recently helped transform the treatment picture for people with cancer. Another form of immunotherapy, called Chimeric Antigen Receptor CAR T-cell therapy, has also generated substantial excitement among researchers and oncologists. Although CAR T-cell therapies are not as widely used as immune checkpoint inhibitors, they have shown the same ability to eradicate very advanced leukemias and lymphomas and to keep the cancer at bay for many years.
Aside from bone marrow derived and adipose-derived mesenchymal stem cells, Esco VacciXcell also provides solutions for CAR T-Cell Therapy.
| Generic Name | Brand Name | Target Antigen | Targeted Antigen | Patient Population |
|---|---|---|---|---|
| Tisagenlecleucel | Kymriah | CD19 | B-cell acute lymphoblastic leukemia (ALL) | Children and young adults with refractory or relapsed B-cell ALL |
| B-cell non-Hodgkin lymphoma (NHL) | Adults with relapsed or refractory B-cell NHL | |||
| Axicabtagene ciloleucel | Yescarta | CD19 | B-cell non-Hodgkin lymphoma (NHL) | Adults with relapsed or refractory B-cell NHL |
| Follicular lymphoma | Adults with relapsed or refractory follicular lymphoma | |||
| Brexucabtagene autoleucel | Tecartus | CD19 | Mantle cell lymphoma (MCL) | Adults with relapsed or refractory MCL |
| B-cell acute lymphoblastic leukemia (ALL) | Adults with refractory or relapsed B-cell ALL | |||
| Lisocabtagene maraleucel | Breyanzi | CD19 | B-cell non-Hodgkin lymphoma (NHL) | Adults with relapsed or refractory B-cell NHL |
| Idecabtagene vicleucel | Abecma | BCMA | Mutliple myeloma | Adults with relapsed or refractory multiple myeloma |
| Ciltacabtagene autoleucel | Carvykti | BCMA | Multiple myeloma | Adults with relapsed or refractory multiple myeloma |
Table 1. Six CAR T-cell therapies have been approved by the Food and Drug Administration (FDA)
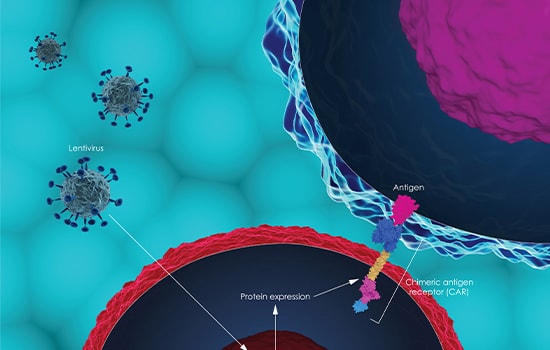
Chimeric Antigen Receptor (CAR) is a new innovative and the first FDA approved therapy that is used to directly target cancer cells. Since 2017, six CAR T-cell therapies have been approved by the Food and Drug Administration (FDA) (Table.1). All are approved for the treatment of blood cancers, including lymphomas, some forms of leukemia, and, most recently, multiple myeloma. CARs are engineered receptors that have undergone genetic transfer technologies that allow T cells to recognize and attach to specific antigen on cancer cells. Unlike chemotherapy, CAR T-cells are unique for they can stay in the body for years, continue to circulate and kill cancer cells.

Esco VacciXcell for autologous cell processing
For some autologous therapies, the cells from the patient are processed on site at the clinic or hospital. These therapies are not regulated as a biologic product and not produced under GMP but rather the devices used are regulated.
Autologous therapies are “custom” products for each patient and the chain of identity of the patient samples is critical to assure the right product is returned to the patient. Scale up of manufacturing for allogeneic cells is similar to techniques used to make protein drugs and other large scale cell derived materials while autologous cells require scale out, the production of many individual products at the same time.
Esco VacciXcell offers various solutions that simplify autologous cell therapy processing using bone marrow and adipose-derived mesenchymal stem cells. The CradlePro-Iso is a cell processing system, includes the CelCradle™ bioreactor system, Esco’s CO2 incubator, Esco’s Versati™ centrifuge, and Esco’s Orbicult Shaker; this one-stop system fully encloses the entire autologous cell therapy process in a cGMP isolator. Due to the CelCradle™ system’s high productivity, a single CelCradle™ bottle can produce an adequate number of mesenchymal stem cells (up to 1 billion) for 1 patient.
The CradlePro-Iso is ideal for hospitals or when working with a few patients per run. Esco VacciXcell also offers cell processing centers to cater to a larger number of patients for autologous cell therapy.
Learn more about CradlePro-Iso.
How Is It Done?
- Patients (Autologous)/ Donors (Allogeneic) collect T cells through a process called Apheresis.
- T cells are isolated and shipped to a processing/ bioengineering facility for manufacturing.
- Since T cells don’t recognize cancer cells or cannot fully destroy all of them in the body, they are genetically encoded using viral vectors to express a specific antigen receptors (CARs) that recognize cancer cells.
- The newly engineered CAR T cells will undergo expansion, concentration and filtration respectively.
- Quality check is strictly enforced before infusion to the patient.
- In preparation for the CAR T, patient will receive chemotherapy to lessen T cells and make room for newly-engineered ones.
- The infusion can be performed either inpatient or outpatient depending on your diagnosis and type of CAR T therapy prescribed.
- The infusion will take about an hour or more and patient will be closely monitored for several hours.

TideXcell supports large-scale LVV production thru efficient cultivation of HEK-293T and subsequent transfection.
For more product information, click HERE
Sources
- Batool Kazmi, Christopher J. Inglefield & Mark P. Lewis. 2019. Autologous Cell Therapy: Current Treatments and Future Prospects. Index WOUNDS. 2009;21(9):234-242.
- National Cancer Institute. 2021. CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. https://www.cancer.gov/about-cancer/treatment/research/car-t-cells





