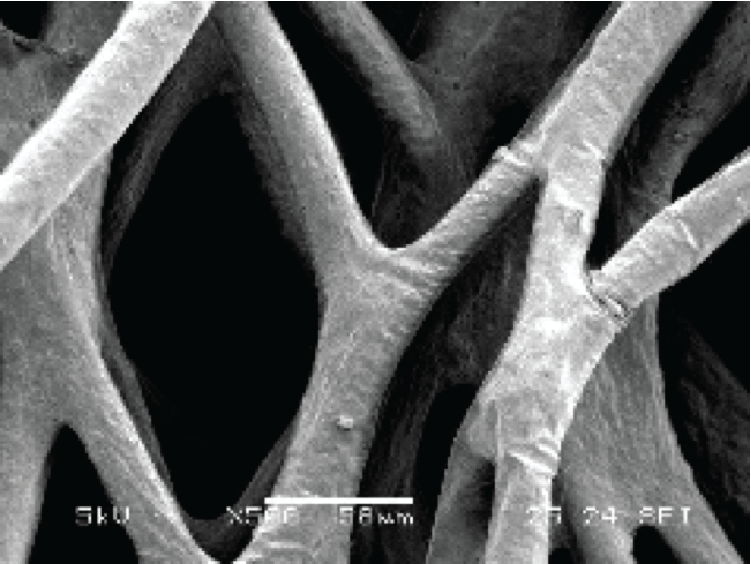
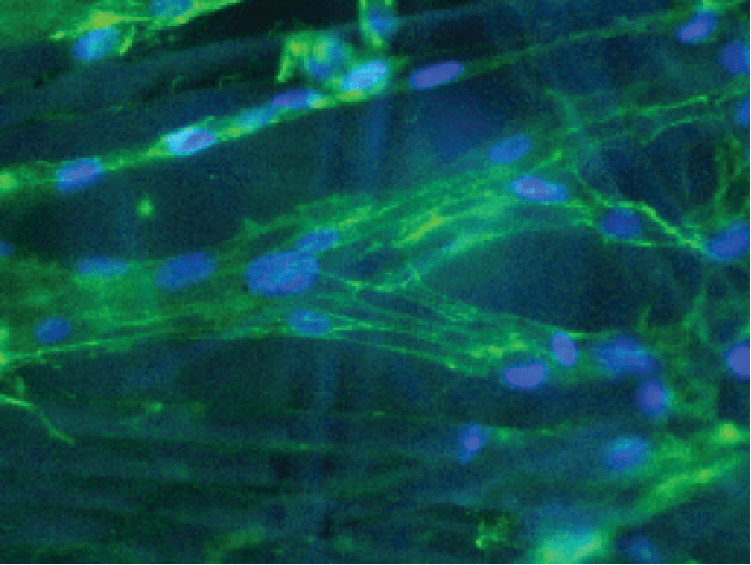
Most isolated cells from animals/humans (except hematopoietic-derived) are adherent in nature. These adherent cells not only represent a formidable production means for viruses or recombinant proteins but also for disease modeling and drug screening in vitro. By contrast, some adherent cell lines were adapted into suspension and do not need surface support for cell growth. Suspension cell lines are usually cultured in flasks that are not surface-treated and require agitation for efficient gas exchange.
Manufacturing vaccines, biotherapeutics, and more involve cell culture (upstream processing) in its early to late stages to produce the desired product. Readily available culture-treated flasks are used for research and development scale in adherent cell culture, while spinner flasks are used for suspension culture. Since adherent scale-up involves an increase in surface area, from simple culture systems like T-flasks and roller bottles, evolved and reliable technologies like packed-bed bioreactors have been introduced. For those that require scale-up for suspension cell culture, traditional stirred-tank bioreactors are used.
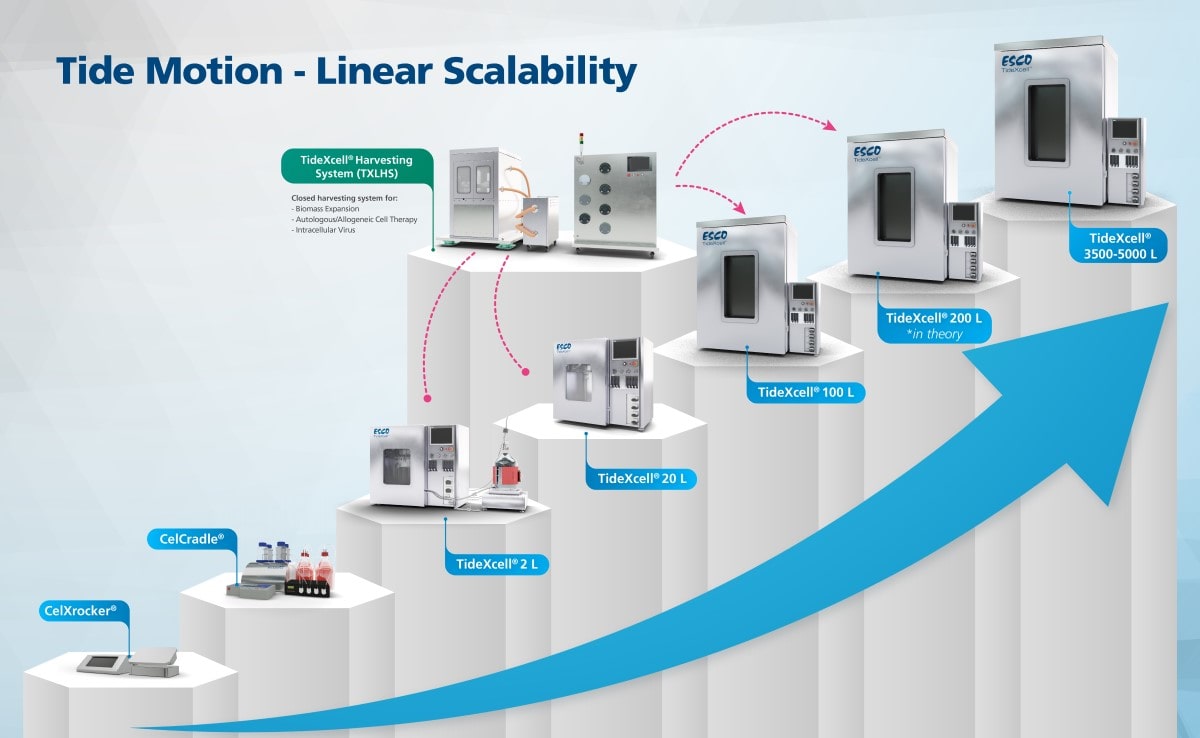
Most applications rely heavily on producing high quantities of adherent cells for vaccine production, cell-, and gene therapies. The current large-scale production of adherent cells requires many pieces of roller bottles and cell stacks, among others. These systems pose challenges due to its labor-intensive, time-, and space-consuming factors. With advanced technologies, one can opt-out from scaling out to scaling up.
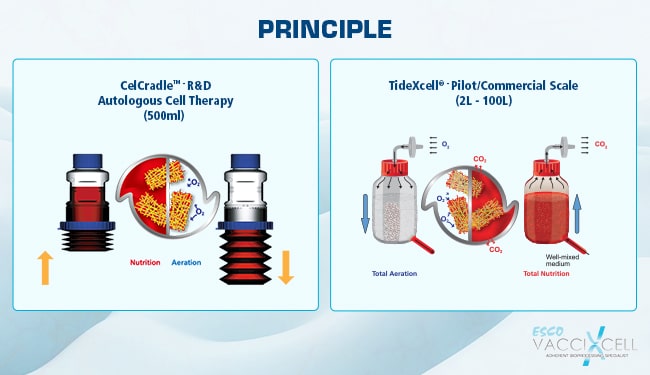
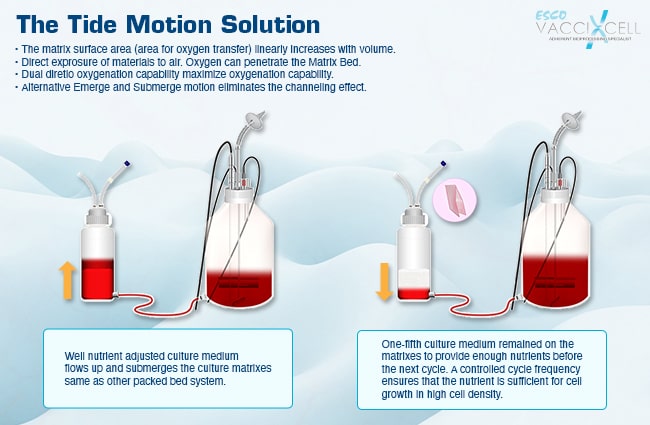
Put bioprocessing in action with Tide Motion. The adherent Tide Motion technology increases your upstream performance with its automated cell culture and harvesting process, reliable scale-up capabilities, and operational simplicity. The bioreactors follow the Tide Motion principle, which is the gentle upward and downward motion of the culture medium in the vessel. This constitutes an environment with extremely low shear stress, high aeration, and nutrition exchange, where adherent cells can grow well and produce high yield products.
Be at ease whether you are culturing adherent cells for vaccine production, cellular agriculture, gene-, cell therapy, monoclonal antibodies, and more.