BioNOC II® Macrocarrier Sampling
Note: Stain to be used for cell monitoring will depend on the cell line used.

Macrocarriers are profoundly used in cell culture for the growth of adherent cells in bioreactors. These vary in morphology, size, porosity, materials, etc. Macrocarriers offer several advantages such as increased production yield due to their capability to provide high surface area-to-volume ratio. Macroporous carrier such as BioNOC II® also creates a 3D cell growth that allows cell to cell and cell to extracellular matrix interactions, mimicking the cell's in vivo environment. The use of macrocarriers allow easier scale-up process that reduces further costs in scaling out to more storage spaces throughout the development.
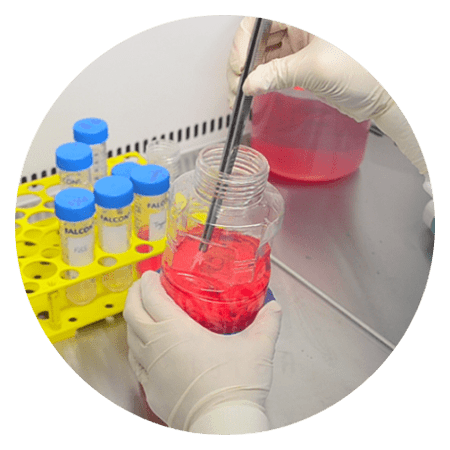
Sampling of macrocarriers and counting of the cells for process development are important steps to monitor the cells’ health and proliferation rate. Consistent results in sampling ensure reproducibility and accuracy. This also allows the assessment of immortalization or transformation, seeding of cells for future experiments, and further use for transfection or infection.
Esco Healthcare’s BioNOC II® macrocarriers allows the growth of adherent cells including animal, mammalian, and insect cells packed in the Tide Motion systems. This macrocarrier is compatible with CelXrocker™, CelCradle™, CelCradle X®, and TideXcell®.
Monitoring the condition of the cells in the BioNOC II® can be done through sampling in the CelCradle™ system. Bring the CelCradle™ bottle inside the biological safety cabinet (BSC) to make sure the sterility of the environment is met. Using pre-sterilized forceps, take out pieces of BioNOC II® macrocarriers from different areas of the bottle and place them in a falcon tube to perform cell growth monitoring.
Figure 2 shows a day 7 mesenchymal stem cells observed under a microscope. The cells were stained with different fluorescence dyes to check the viability of the cells in the carriers. When the cells have grown to maximum confluency, the cells can be seen protruding from the edge. The cells have secreted extracellular matrix, indicating that the cells have actually achieved maximum growth in the BioNOC II® macrocarriers.
Cell staining is a technique used to visualize cells and other cell components using a microscope. This process may involve immersing the sample in a dye solution and then rinsing and observing the sample under a microscope.
Note: Other types of dyes may be used, eg. Trypan blue. Use fluorescence dye for staining to obtain clearer cellular morphology.


Figure 4. Cell staining on BioNOC II®
Figure 4 shows a comparison of stained BioNOC II® macrocarriers from Day 1 to Day 3. Cells have evidently propagated on Day 3.
Note: Other types of fluorescence dyes can be used to visualize the cells. Eg. fluorescein diacetate, cell tracker, etc.
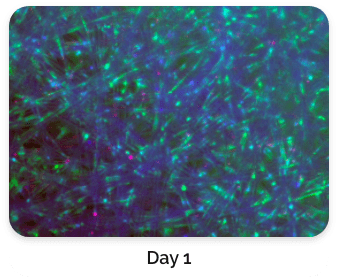
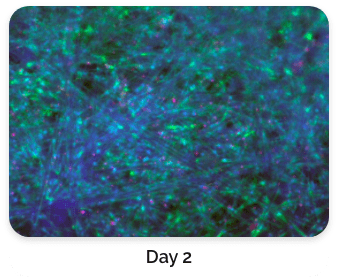
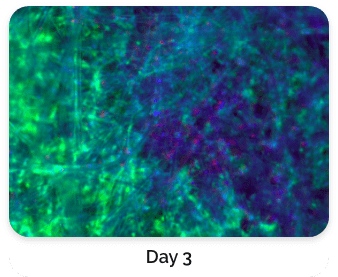
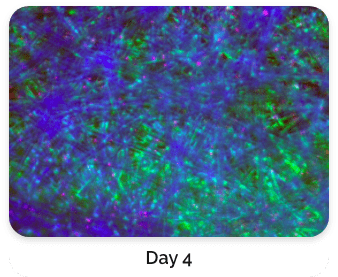
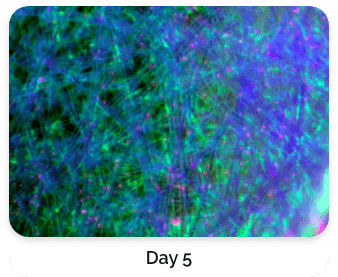
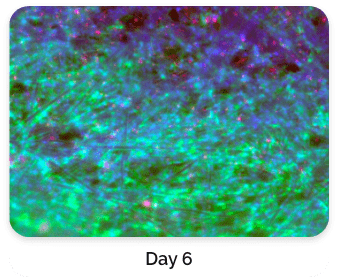
Figure 5. Green: Fluorescein diacetate (cytoplasm), Blue: Hoechst 33342 (nucleus), Red: propidium iodide (dead cells)
For this type of process that relies on macrocarriers, the cells are typically released by exposing them to an enzyme for a short incubation period. It is very crucial and expertise is needed to find the right concentration of enzyme and incubation time to not damage or kill the cells due to over-exposure.
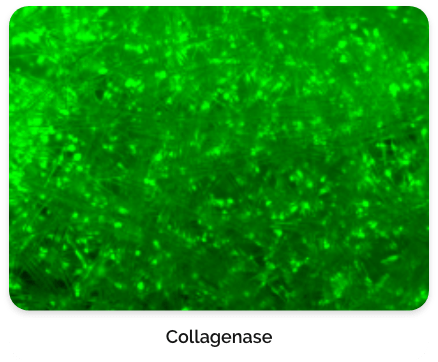
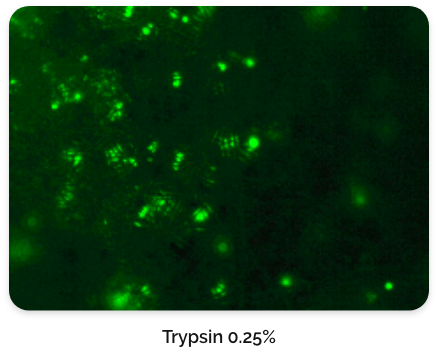
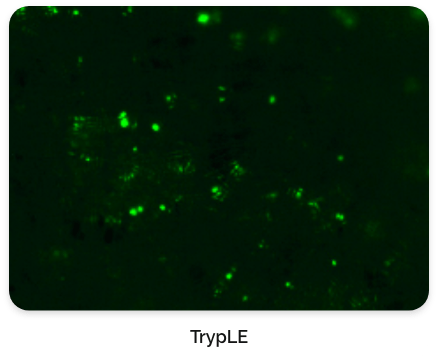
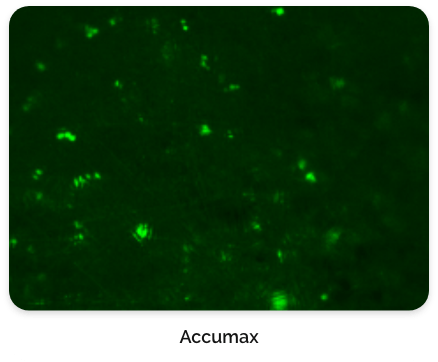
Figure 6. CL-MSC cells left behind after harvest.
Cell counting plays a major role in assessing cell viability. Knowing the number of viable cells is crucial for standardizing the succeeding phases of the experiments. This is important especially for downstream experiments that require a specific, accurate, and consistent number of cells for the next steps of the process. For cell culture, measuring the number of cells grown in the carriers is essential to determine the level of confluence before passaging for optimal cell growth.
Hemocytometers are made of special optical glass on which cell suspensions are loaded in specified volumes and counted under a microscope. Hemocytometers consist of two counting chambers, each of which is divided into nine large 1mm squares, on an etched and silvered surface separated by a trough.
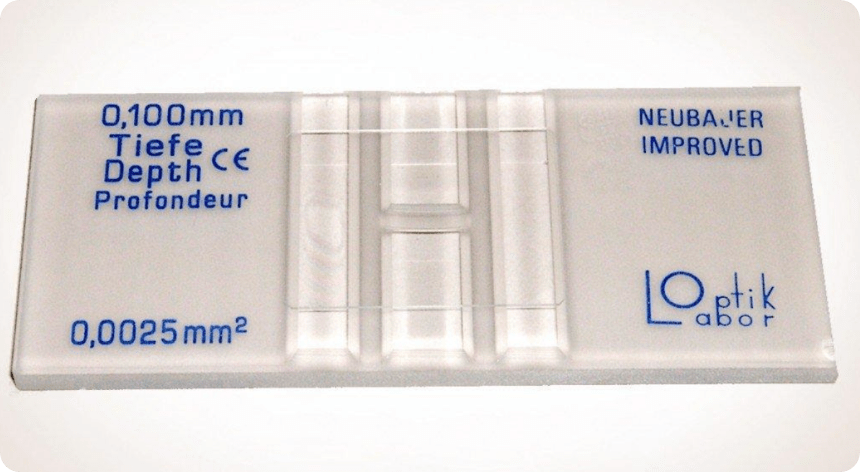
Figure 7. image of a hemocytometer
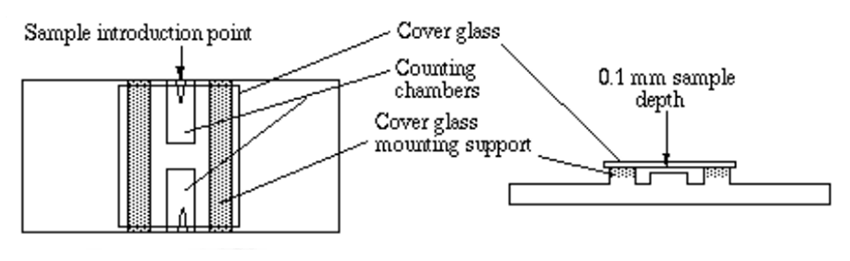
Figure 8. Parts of a hemocytometer
Place the hemocytometer under the microscope in such a way that the first small square of the top big square is visible in the middle of the field of view.
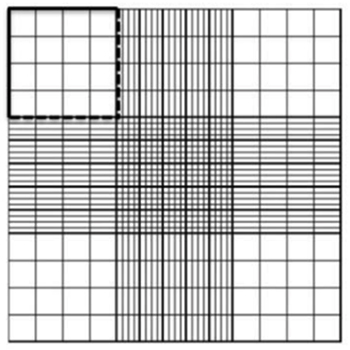
Once the cell numbers are obtained in the first square, proceed to the second (the one on the top right) and repeat the process. Remember, counting procedures should be consistent. If counting started with the cells on the top and right sides but not the ones on the bottom and left sides of the first square, then this must also be done on the second one.
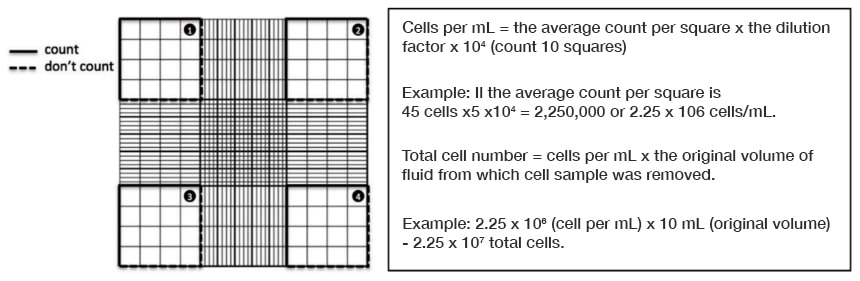
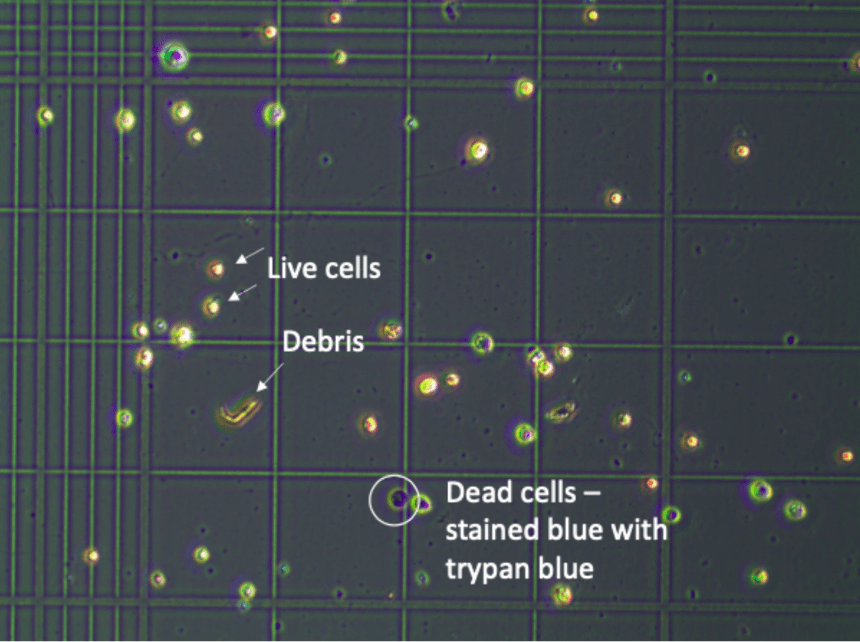
Figure 9. Example of cells on hemocytometer
Sign up to our newsletter and receive the latest news and updates about our products!
