Applications
Cell-based Influenza Vaccines
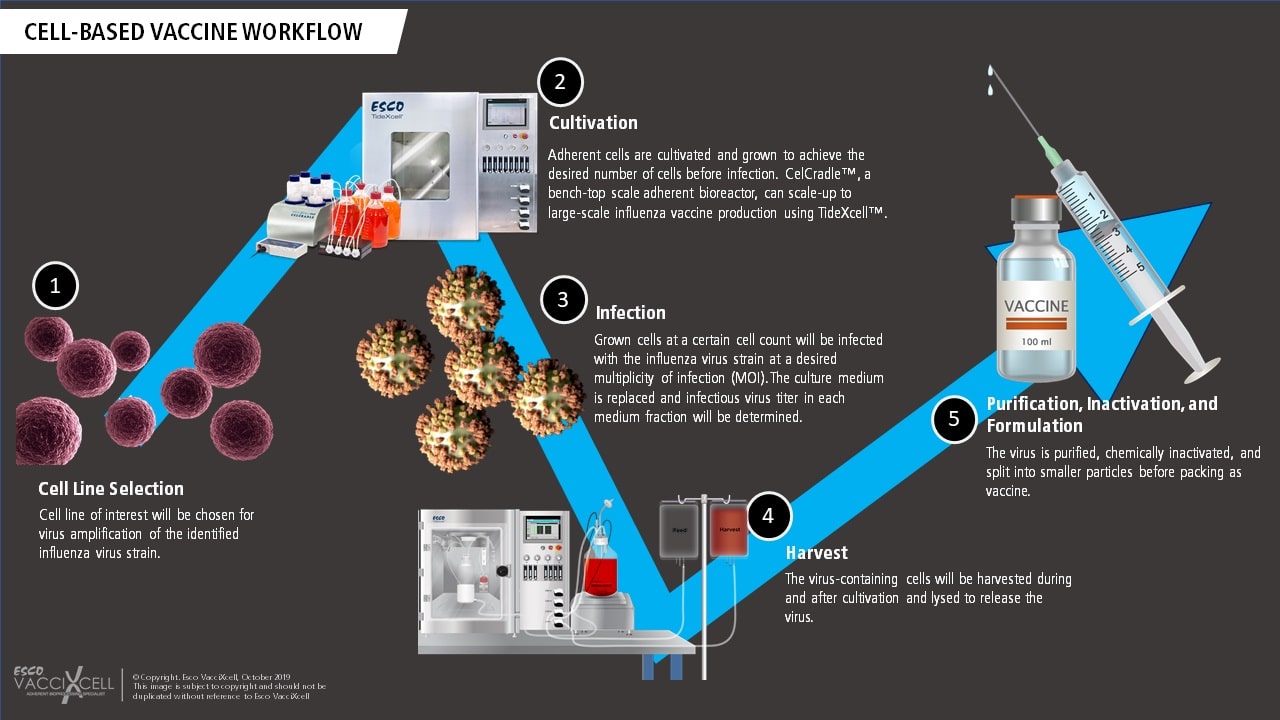
Cell-based vaccines such as the use of VERO, MDCK and recently PER.C6 cell lines for viral production involve a controlled and adherent scale-up, closed manufacturing process. Influenza viruses, for example, are grown in cultured mammalian cells using two-dimensional (T-flasks, cell stacks) or in three-dimensional (bioreactors with microcarriers or macrocarriers) systems. This process is deemed flexible and provides faster timeline due to adherent scale-up using bioreactors. When producing live-attenuated cell-based vaccines, the adherent cell line of interest is chosen and screened for its virus amplification capability.
The chosen adherent cells are then cultured in an in vivo-like environment before reaching confluency and infected with the virus. Tide Motion bioreactors are usually used for this application to produce high viral titer yield. The cultivation process continues for a few more days, afterwards, the virus-containing cells/fluid are harvested, inactivated using a highly stringent procedure (sometimes even split into smaller particles to further prevent replication), and purified to become a vaccine. The workflow for cell-based vaccine production is depicted at the provided.





