Applications
Cellular Agriculture
Production of animal proteins and cells through culture methods have been developed to reduce the impact of livestock and poultry industries on the environment, animal ethics, and human health.
The goal of cellular agriculture is to provide animal proteins through sustainable production with minimal resource input and environmental impact.
Both acellular and cellular products are being produced through cellular agriculture methods. Livestock emerges as one of the direct emissions contributors of global greenhouse gasses about 10%-12%. This matter is also getting serious when massive efforts to feed people every day with livestock products increase as the world population becomes greater. Over the past 50 years, global meat consumption has risen substantially, surpassing 350 million tons in 2023 (World Animal Foundation, 2024). Providing food for an increasing population will be a great challenge that coincides with the pressure to reduce negative environmental impacts of conventional agriculture. These two are among many reasons behind the first coining of cellular agriculture in 2015.
Cellular Agriculture In a nutshell:
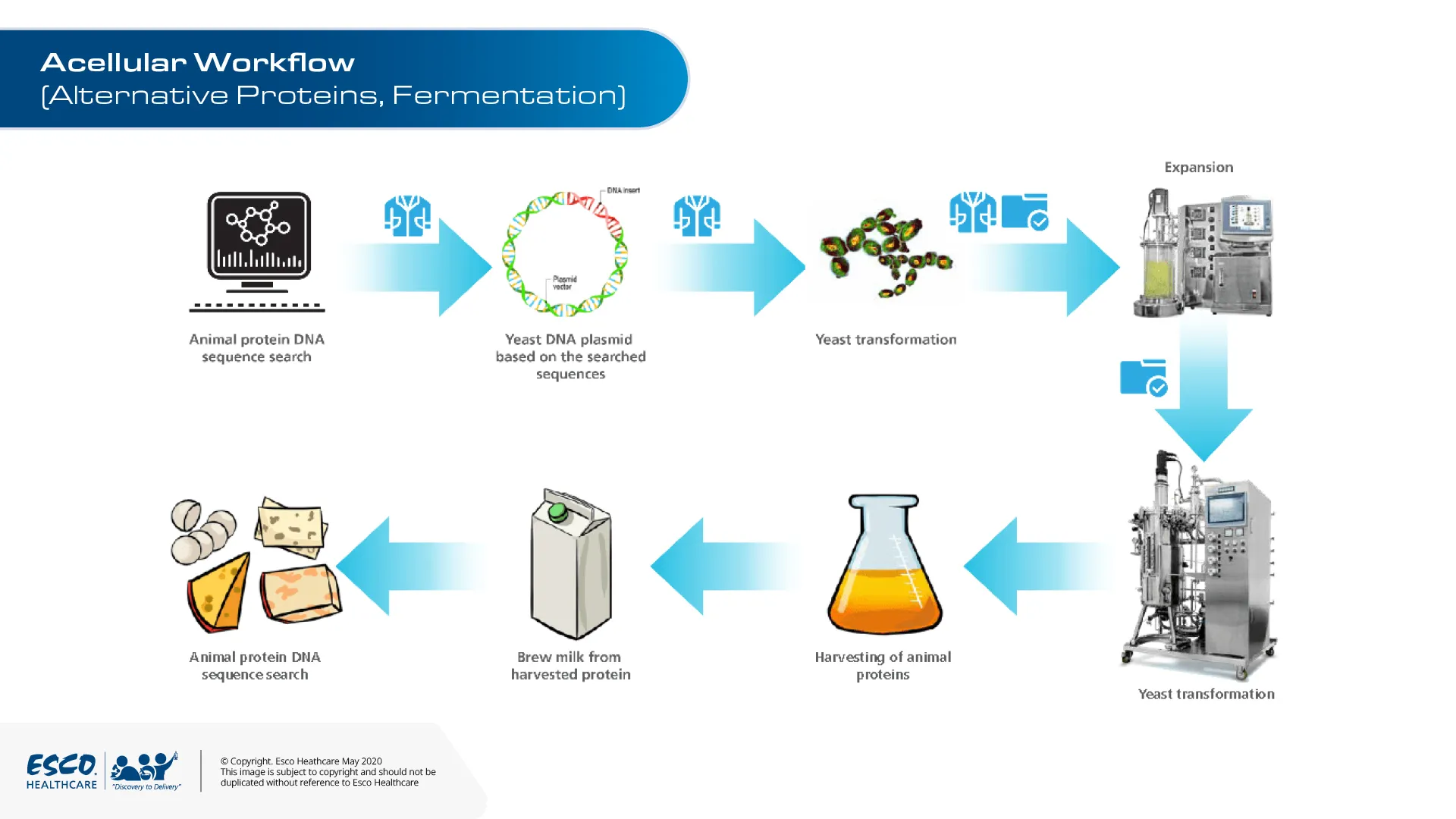
Cellular agriculture refers to the production of animal products through culturing methods. These products include whole cell (cellular product) and also animal biomolecules, such as proteins and fats (acellular product). Cellular products encompass tissue engineering, and cultivated or cultured meat.
Esco Healthcare Tide Motion bioreactors provide a single-use capability and process monitoring technologies to ensure that the resulting cultured cells are manufactured under robust conditions.
Deep Dive: What is cellular agriculture?
Cellular agriculture is a technology for growing animal agricultural products through cell cultures instead of raising livestock. Carrying the foundation of a biobased economy, cellular agriculture upholds animal welfare, food security, and human health. Through its biotechnology, cellular agriculture is considered to have zero substantial global problems of detrimental environmental impacts caused by raising livestock.
The idea of cultured meat as an alternative to conventional meat was originally envisioned by Frederick Edwin Smith and Winston Churchill in1930s (Arshad et al., 2017). The term cellular agriculture was first coined in 2015 by Isha Datar, executive director of US-based 3rd sector group New Harvest. Potential future products bracketed under the label cellular agriculture include meat produced through tissue engineering (variously known as cultured meat, clean meat, cell-based meat and cultivated meat, and animal-derived products such as milk, leather and egg white produced through recombinant DNA fermentation.
This is how cellular agriculture offers a solution
Cellular agriculture is a new application of producing agricultural products such as food or materials via bioprocessing methods in the laboratory. It can be any product like eggs, meat, milk, and many more. Through cellular agriculture products such as laboratory grown meat, the world saves 99% land, 82-96% water, and 7-45% energy compared to raising livestock. In this new innovative method, we can address the food shortage to feed the growing population of the world.
One of the most known concepts of cellular agriculture is cultured meat, animal carcass or biopsy. This can be done through mechanical means like scraping or manually slicing off pieces of tissue or through biopsy needles that are inserted into the body to extract tissue samples. The cells are then cultured with nutrients and growth factors to produce muscle tissue which can be harvested after about three weeks when it reaches the desired size. It is possible to grow cells from different types of animal tissue and turn them into edible tissue. The process for creating cultured meat starts with harvesting animal cells from an animal carcass or biopsy. This can be done through mechanical means like scraping or manually slicing off pieces of tissue or through biopsy needles that are inserted into the body to extract tissue samples. The cells are then cultured with nutrients and growth factors to produce muscle tissue which can be harvested after about three weeks when it reaches the desired size. It is possible to grow cells from different types of animal tissue and turn them into edible tissue.
There are several companies which produce cellular meat (CM) worldwide. Among the highest profile in the US are Memphis Meats, who were the first CM company. Others include Mission Barns, Wild Type, and Bluefin tuna-focused Finless Foods in America and Mosa Meat (Netherlands), Meatable (Netherlands) , Future Meat Technologies and Aleph Farms (Israel), Integriculture (Japan), Shiok Meats (Singapore), and Appleton Meats (Canada).
Types of Cellular Agriculture Products
Acellular Products
In making cheese, acellular products include casein, a milk protein, and vanillin, an ingredient for making cheese.
Production of milk protein can be achieved through recombinant DNA technology. Microbes such as yeast can be engineered to produce casein, chymosin, and kinase which are important in the production of cheese.
Acellular agriculture falls under the general niche of cellular agriculture, which is the production of animal-based products in a lab environment. As opposed to taking resources like milk directly from the cow, acellular agriculture allows us to utilize the process of fermentation. Fermentation process is utilized to maintain the ideal parameters for protein production. These proteins are then purified and used to create products such as cheese, milk, or any other dairy product.
One of the important milk proteins of an important source for babies is breast milk. Breast Milk contains immunoglobulins that is one of a crucial immune protective component. Babies who are breastfed have a passive immunity for longer.
Although no one can deny the benefits of breastfeeding, some mothers encounter challenges related to it including bleeding, sore nipples, cluster feeding, and poor attachment. This forces them to resort to artificial and commercial milk sourced from animals that are not of the same value with human breast milk. Sugars, fats, and immune cells are lacking in current commercial formulas. Human milk also varies for each mother, and thus each baby has its own customized milk. Previous infection of the mother can generate antibodies that studies have found to pass to her milk, including COVID-19 infection as some studies suggest.
Aside from breastfeeding concerns, milk from cows is sourced from cattle farming which can be difficult to achieve under high-temperature regions such as the tropics. Also, contaminants may be found in the milk when antibiotics are used for treating mammary glands infection. Other alternatives such as plant-derived products have been in development but may not have the nutritional content that is needed for babies.
Challenges of this novel dairy milk production include manufacturing consistent composition in terms of production for the dairy market as well as quality monitoring different from the current QC techniques for dairy products. To help the cellular agriculture industry, incubator programs such as Milk Cubator, by Pascual Innoventures, have been launched targeting innovation by start-ups. These start-ups would have access to food experts as well as established facilities to ramp up product and technology development.
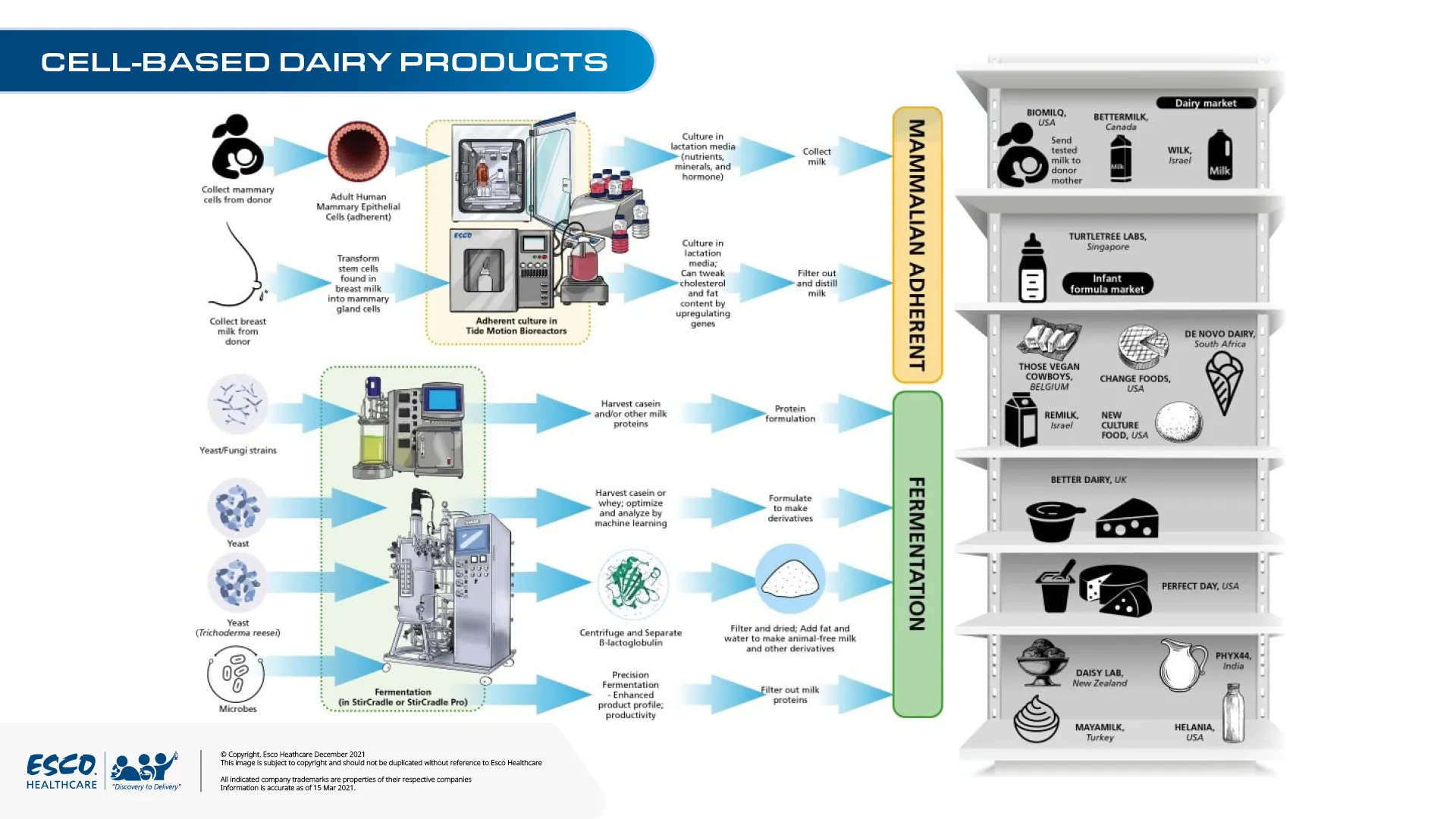
Aside from cell-based dairy products, other technologies such as Moolec Science hybrid plant and cell-based proteins are also entering the development of animal-free dairy and egg options. They use genetic engineering of animal proteins into crops that would be grown and harvested for a carbon-neutral and animal-free process.
These budding cell-based dairy products are proof of the use of cell-based technologies for the production of nutritionally important products. However, these are still in early, infant, stages and immunologically, current cell-based breastmilk is still not considered replacement as it is still missing immunological and hormonal components from breastmilk. To date, cell-based breastmilk is considered a supplemental nutrition aid.
Cellular Products
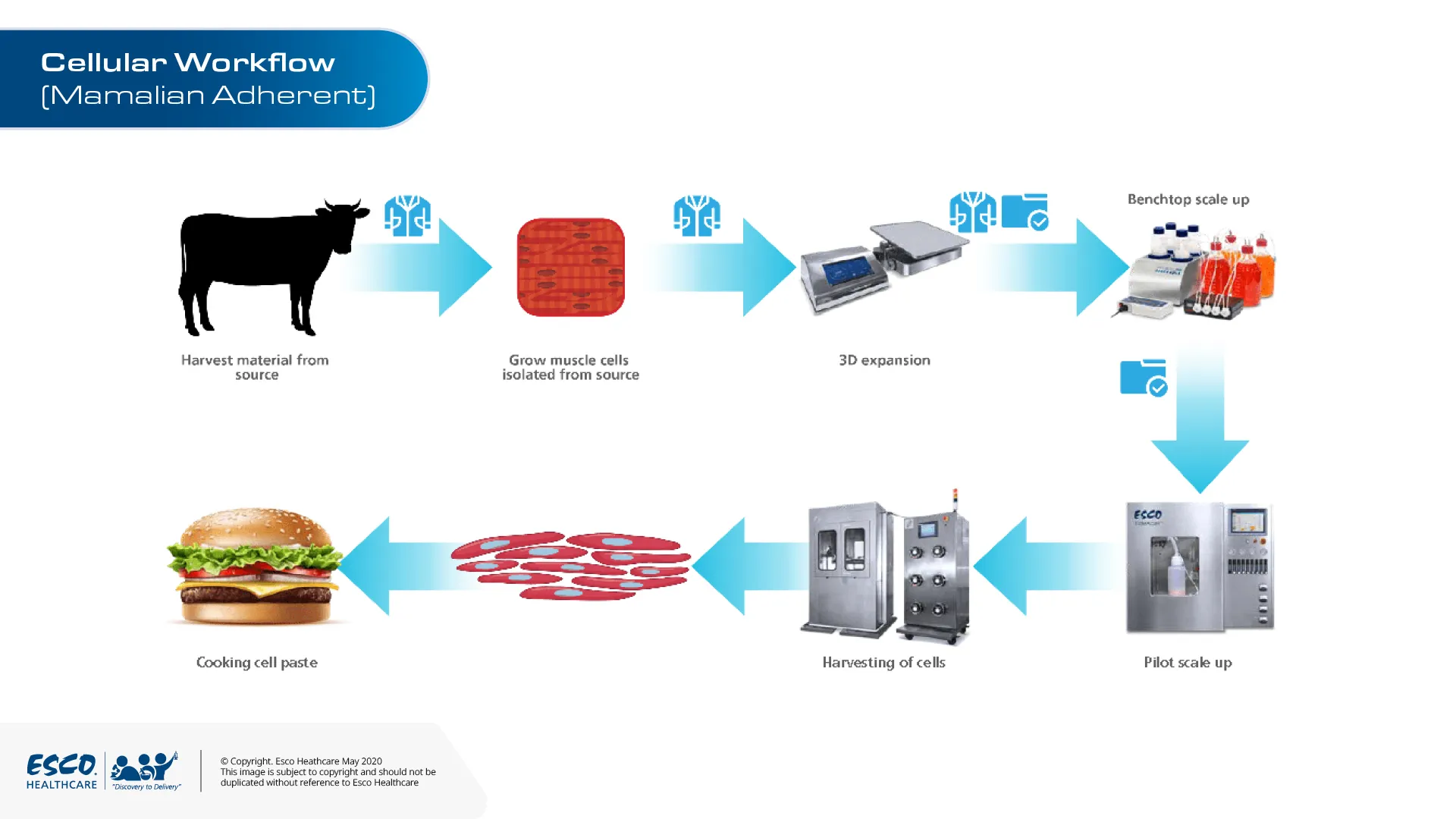
Cellular products include tissue engineering, and cultivated or cultured meat.
Cultured meat refers to the application of cultured methods to produce muscles that are rich in protein for human consumption.
Meat is mainly represented by skeletal muscles located at different body regions (carcass). Decades of cell culture of muscle cells, adipocytes, and fibroblasts for research purposes brought the prospects to exploit the collected knowledge for artificial meat manufacturing.
Tissue engineering methods have been used to culture muscle cells mimicking in vivo environments. A scaffold is required to allow adhesion, proliferation and maturation of the muscle cells. For non-edible scaffolds, however, cultured muscle cells are separated first prior to harvesting; since this procedure might damage the muscle cells, protective techniques such as thermoresponsive coatings are used.
Cellular agriculture is the production of food products with the use of cell cultures. Instead of raising livestock and animal slaughter. Scientists can grow animal products in the laboratory through cell cultures. Through biopsy, we can collect any cell from animals. These cells can be programmed as iPScs which differentiated into muscle cells and other cell types. It can be a mixture of cells depending on how the meat shall be designed and developed.
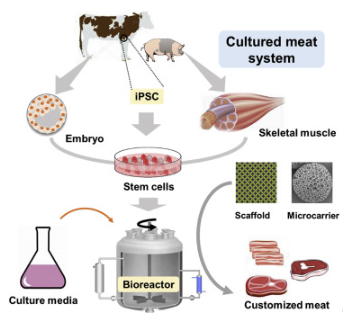
Figure 1. Cell samples obtained from animals can be differentiated into different cell lines. One of these cells is induced pluripotent stem cells (iPSCs). Laboratory grown meat can be sourced from the cells of a chicken, cow, ducks, pig or any other desired animal.
TideXCell® is equipped with BioNOC II® macrocarriers, where desired cells can be proliferated to maximum. By starving the stem cells through 100% media exchange, they differentiate, maturing into skeletal muscle cells that then merge into primitive fibers.
Potential Benefits of Cultivated Meat
The advantages of cultured meat are plentiful. It is much more sustainable than traditional factory farming because it doesn't require the use of land or water resources, it's cheaper than most meats on the market today (it's estimated that cultured beef will be priced at $2 per pound) which means people from all walks of life will be able to afford it, and it has potential to end world hunger.
Zero animal cruelty
There are over 70 billion animals that are farmed annually around the world. Approximately 50 billion of these animals were treated like machinery more than living, breathing animals. Many animals experience short miserable lives in their cages, crates or pens rather than living in their natural behavior.
Less environmental impact
As there is an increase in demand for meat products, a lot of forest land was converted into livestock barns, ranches, and stables. Whereas in cultivated meat, it only requires small or less land for the production, less water and reduces pollution. One example is the production of traditional beef which requires a lot of carbon dioxide, methane and nitrous oxide gasses that contribute to global warming. Cultured meat can reduce these types of emissions globally.
Health Benefits
Every day of consuming meat can lead to some chronic disease especially if these food products did not go through any quality assessments. In cultured meat, producers can adjust the amount of cholesterol or fats that should be included in these food products such as burgers, nuggets, sausage, or steak.
Cultivated meat producers may replace the saturated fatty acids with omega-3 fatty acids which can be healthier protein alternatives.
Hence, there is no clear evidence yet or what other impact does cultured meat have on nutrition.
Does not require antibiotics
One emerging threat to human health is the overuse of antibiotics in livestock farming. This can build up antibiotic resistance wherein many common infections will no longer have cure or treatment, thus, later on will kill many animals. These drugs will be put into waste as it will no longer work to treat the infections
As in cultured meat, it will be produced in a clean site to have lesser bacterial infection such as E.coli, which can be found in animal manure, and other contaminants that you can find in a traditional meat plant.
Does not require growth hormones
In other countries like the United States and Canada, cows were given hormones to produce milk and grow larger than usual. On the other hand, researchers in Europe banned these hormones as it may cause post health risk to humans. So cultured or cultivated meat requires non-growth hormones in order to have a huge amount of meat.
Low-cost production than the traditional meat processing
Cultured meat or these alternative proteins is currently $2000 to produce a single pound of meat, thus, people won’t be paying more than $500 for a quarter pounder. Consumers should not be worried as it is just the same as other prototypes that are always expensive in the beginning before they become very affordable. The first ever cultivated meat was created a few years ago with a production cost of more than $300,000 dollars. Because of this, meat companies are working out to scale up in the production as there's already a massive reduction in the operational expenditures. Therefore, these alternative proteins or cultured meat should be eventually comparable to traditional meat.
Sustainability to reduce global hunger
According to researchers, almost 21 million or 11% of the world’s population is already suffering from undernourishment while 821 million is suffering from constant hunger. Hence, it is very vital to embrace new technologies in order to address these challenges. With cellular agriculture, there is an increase in an accessible and sustainable high quality meat supply for many people across the globe at a lower environmental cost.
Still, there are several factors to be considered as to whether one will accept or reject cultured meat. Consumer’s perception is still debating the ethical, naturalness, and religious considerations for eating cultured meat.
Esco Healthcare Tide Motion Bioreactors
A variety of methods can be done for scaling up cellular agriculture products from benchtop to pilot and manufacturing scales. Esco Healthcare Tide Motion bioreactors have the capability to provide an end-to-end process without compromising the quality and quantity of these cultured meat. For laboratory scale we have CelCradle® and TideXcell® for pilot and manufacturing scale. These tide motion bioreactors are equipped with BioNOC II® macrocarriers, where desired cells can be proliferated into maximum density. By starving the stem cells through 100% media exchange, they differentiate, maturing into skeletal muscle cells that then merge into primitive cells.
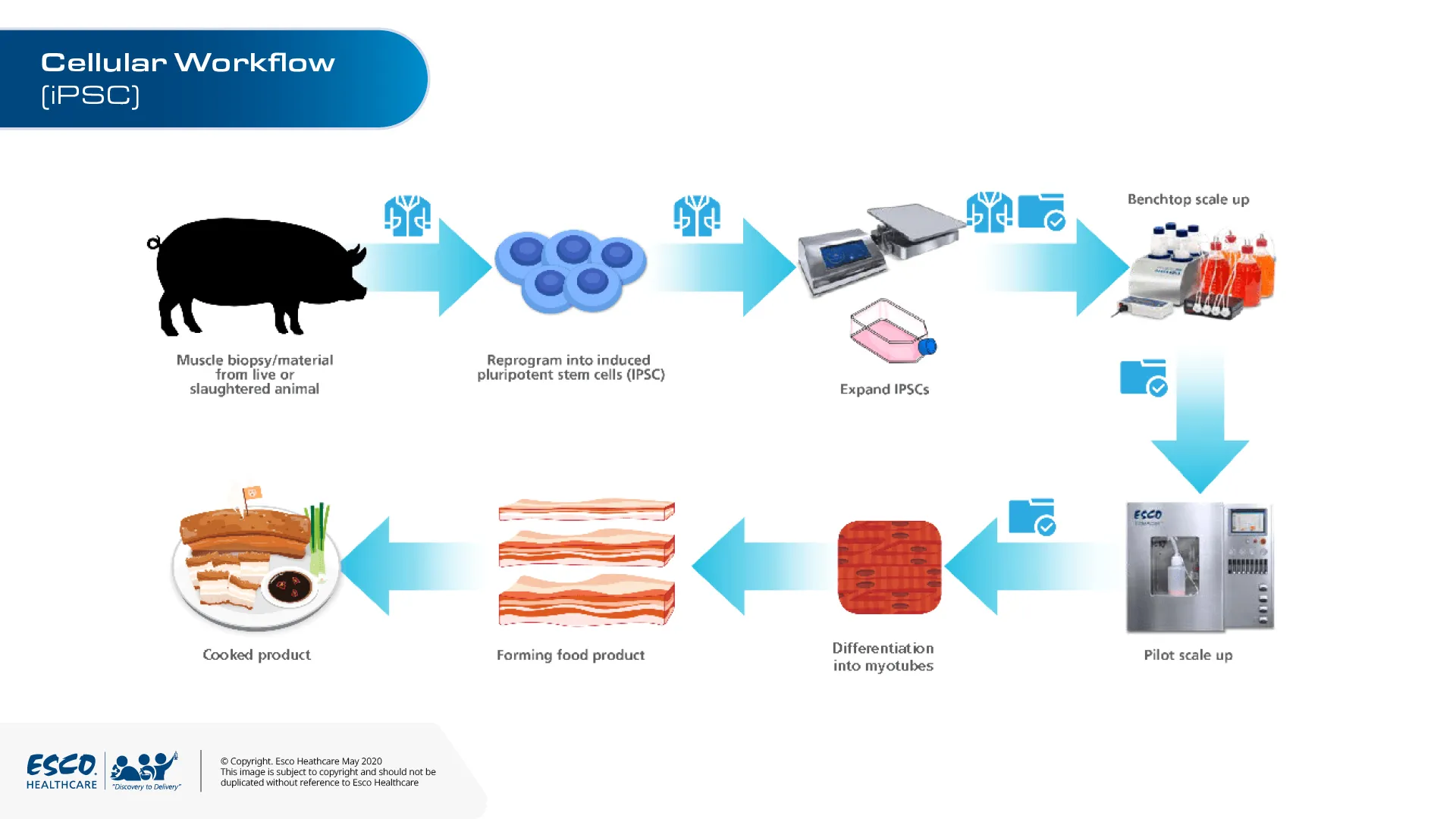
Figure 2. An example of scale-up strategy for IPSCs based meat products. iPSCs derived cells were expanded in T-flask to determine an appropriate seeding density for BioNOC II® in CeXrocker™ with an appropriate culture media to keep its pluripotency. When the target number of cells is achieved, it will be transferred and expanded in the CelCradle® system.





