
Genes are the basic hereditary units that define a person’s traits – the features or qualities that are inherited from the parents. They carry the information that makes a person unique such as hair type, eye color, height, and even the smile. These physical attributes come from one generation and pass on to the next through genes.
Genes comprise DNA. Over the lifespan of an individual, DNA mutations are bound to happen. These are generally influenced by mistakes in DNA replication or driven by environmental and carcinogenic substances. Any random, unexpected changes that occur in the DNA of a gene may result in protein malfunction that is harmful to the health. These mutations are likely to cause certain diseases such as genetic disorders or cancer.
A promising solution to the problems of mutations is a technique called gene therapy. This technique is used to engineer gene expression or alter the biological properties of a living cell to prevent or treat diseases. Gene therapy allows scientists and doctors to treat diseases through the insertion of an engineered gene into the patient’s cell.
Gene therapy research has been around for over decades, and its potential serves as a silver lining to many people. The main advantage of gene therapy that outweighs its disadvantages is the promise of providing a patient with a genetic disease or cancer, a life free of regular therapies and disease management.
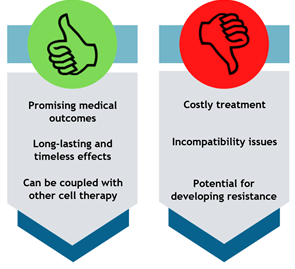
Genes that are inserted directly to the cell are likely to degrade. Rather than direct insertion, vectors are used to operate as carriers to deliver the genes. Vectors are defined as any vehicle used to transport a specific DNA sequence into the host cells.
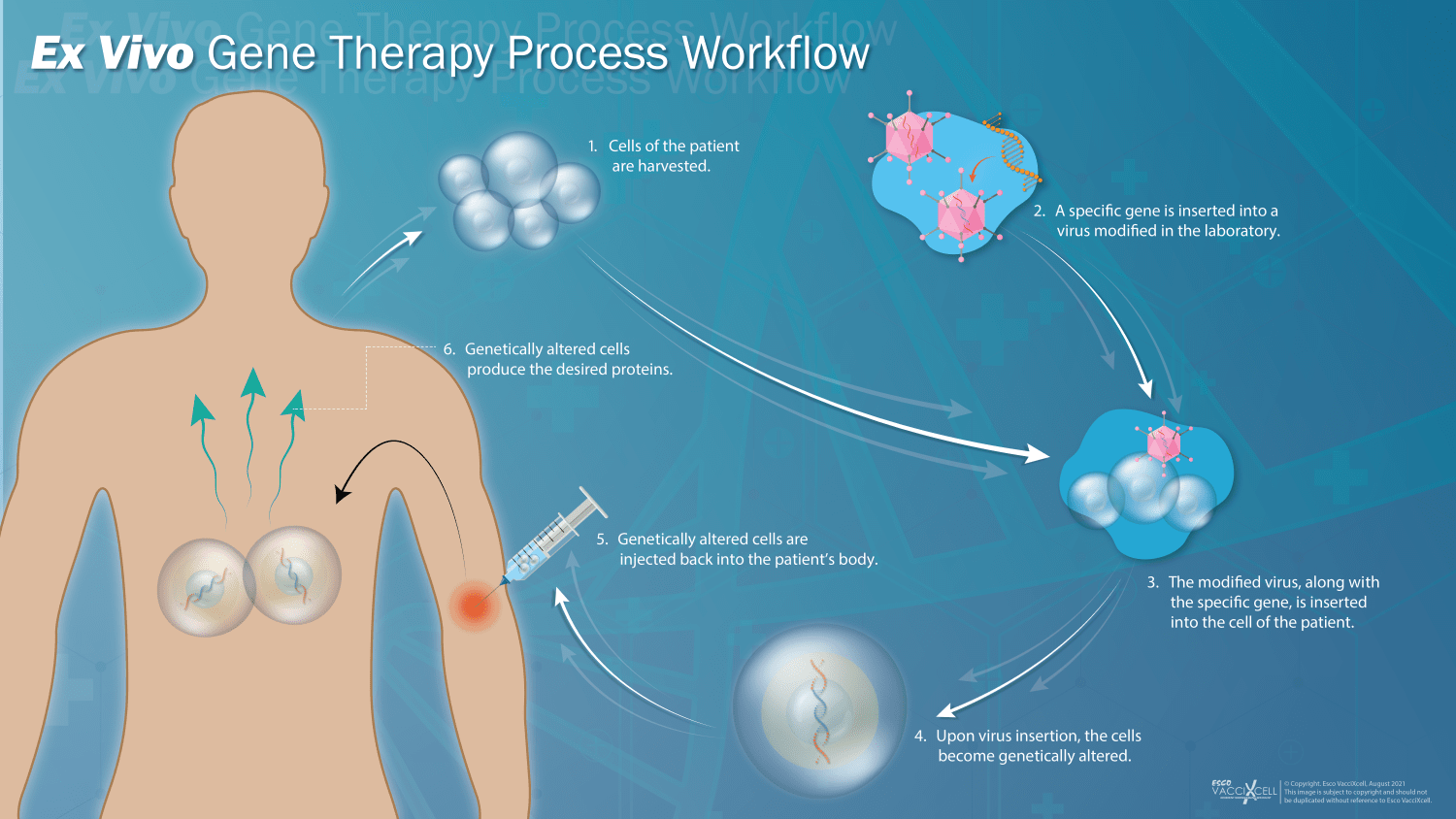
Figure 1. 5-step general process of ex vivo cell therapy.
Cell therapy begins with harvesting the stem cells and T cells from a patient. Meanwhile, a virus is genetically engineered in the laboratory to remove certain genes that can potentially cause a disease, lowering its pathogenicity. A specific, therapeutic gene is then inserted into the genetically engineered virus. The altered virus, along with the gene, is inserted into the harvested cells turning them into modified cells. The modified cells are infused back into the patient’s body to target and kill the antigen-expressing cancer cells. On the contrary, in vivo gene therapy requires direct administration of the therapeutic gene into the viral genome. The viral vector containing the therapeutic gene is then injected back into the patient’s body, producing the desired proteins encoded by the therapeutic genes.
These two gene therapy techniques both have their own advantages and limitations. Ex vivo gene therapy has low immunogenicity and offers the capability to evaluate transduction efficiency before infusing the cells back into the patient’s body. In vivo gene therapy is usually preferred by scientists; however, the drawback of this strategy is its need for high specificity in targeting only the desired cells and tissues. Poor targeted delivery induces an immune response that may pose risks to the patient.
Viral vectors utilize viruses as tools in delivering genetic material into a target host cell. Viruses are used due to their natural capability of entering the cells in the body. However, these viruses are modified in a way that they could not cause a disease to the patient who receives it. Retrovirus, lentivirus, adenovirus, and adeno-associated virus (AAV) are among the viruses that have been used in laboratories for their gene therapy applications. Each of these vector systems has a distinct application best suited for the functions it holds.
Table 1. Commonly used viral vectors in gene therapy and their features
| Vector | Features |
|---|---|
| Adeno-associated virus (AAV) |
|
| Adenovirus |
|
| Retrovirus |
|
| Lentivirus |
|
Adeno-associated virus is among the most studied vector vehicles in gene therapy. Initially, AAVs were derived as a contaminant in adenovirus preparations. AAV is a capsid (protein shell) that functions to protect a small, single stranded DNA. There are several serotypes of AAV, however the majority are based on AAV serotype 2 (AAV2). The life cycle of AAV in human cell begins by binding to a receptor present on the surface of the cell and its subsequent entry into the target host cell by a cellular process called endocytosis. Once the virus is engulfed, it escapes from the endosome and enters the nucleus wherein it promotes transgene expression. Some of the advantages of AAVs include superior biosafety rating as it is known not to cause diseases, low immunogenicity further supporting its lack of pathogenicity, wide-range cell transduction, and stable and long-term expression.
Adenoviruses are the most common causes of typical illnesses such as colds, fevers, flu, and more. These viruses are known, in most cases, to not cause severe and life-threatening infection, making them an ideal vector for carrying genes to the target host cells. Adenoviral vectors comprise a double stranded DNA genome enclosed in an icosahedral nucleocapsid. Their entry into the cell involves a series of interactions among the proteins on the surface of the vector and the target host cell. The transduction occurs via the absorption of the virus onto the target cells through the coxsackie and adenovirus receptors (CAR) present on the surface of the cell. These viral vectors then introduce their DNA molecule into the host, however, do not incorporate their genetic material into host cell’s genetic material. Instead, their DNA molecules are released in the nucleus of the cell, and are transcribed like any other cell, but do not replicate upon cell division.
Lentivirus belongs to the Retroviridae family and is known as a subtype of retroviruses. The pathogenesis of lentivirus is similar to that of retrovirus. Their genome comprises RNA that is reverse transcribed into DNA, replicated in the process, and integrated into the host cell’s genome. Once it integrates, the host cell transcribes its genome along with the viral genomes, resulting in a stable transgene expression. The main difference among these two viral vectors is lentiviruses can infect both non-dividing and dividing cells, while retroviruses are limited to infecting only the actively dividing cell types.
“Retro”-virus implies a backwards behavior contrary to the conventional concept of genetics, which starts with DNA replication, transcription, and translation. The RNA genome of retroviruses is contained in a glycoprotein envelope which interacts with receptors on the target host cells to gain entry during the onset of infection. Once inside the cell, reverse transcription occurs as the single stranded RNA of the virus is converted into double stranded DNA. As the cells undergo division, the viral DNA integrates with the target host cell DNA. This stable integration is considered the most significant advantage of retroviruses from other retroviral vectors.
Non-viral vectors do not integrate viruses in their delivery systems, therefore, are less likely to cause diseases as compared with viral vectors. Instead, physical, and chemical methods are the two ways these vectors carry out the delivery of the genetic material into the host cell. Physical methods are used in delivering genetic material to any target cell with the means of force in order to disrupt the cells’ membrane barrier, allowing its entrance into the target host cell. Physical methods generally include direct microinjection, electroporation, plasmid injection, and ballistic injection of DNA. On the other hand, chemical methods usually involve carriers such as lipids, polymers, and peptides which have cell-specific functionalities that mainly affect specific cells.
Table 2. Commonly used nonviral vectors in gene therapy and their features
| Chemical Method | Features |
|---|---|
Liposomes
|
|
| Polymers |
|
| Peptide-DNA Complexes |
|
| Physical Method | Features |
| Microinjection |
|
| Electroporation |
|
| Biobalistic |
|
Liposomes are characterized as spherical vesicles that serve as vehicle for drugs and small molecules to be released in target site in the body. These vesicles are deemed versatile due to their dynamic properties that are simple to manipulate, therefore making them an ideal delivery system for therapeutic purpose.
Over the years, polymers have been widely studied and developed for a wide range of biomedical and therapeutic applications due to their versatile conformations, biodegradability, and ease of synthesis. The first variety of nonviral gene delivery systems are cationic polymers, and these are discovered to interact with negatively charged DNA to form complexes, known as lipoplexes. Lipoplexes have the ability to shield genes from degradation and transport them inside the target cells.
The main goal of peptide-DNA complexes in gene therapy is to mimic the function of viral proteins through short peptide sequences of approximately 50 amino acids or less. Besides their biodegradability and biocompatibility properties, the amino acids in the sequences can be easily manipulated to enhance their capacity to overcome the barriers for gene delivery. Additionally, peptides are fairly easier to produce and store as they are stable under ambient conditions.
Exosomes, termed the ‘rising star’ in drug delivery, are presently recognized to have a major role in mediating cell-cell communications. These are fluid-filled sacs extracellular vesicles that are secreted by cells as means of communicating with other cells in the human body. In fact, exosomes are found in all bodily fluids such as blood, saliva, urine, etc. Exosomes are excellent and feasible gene delivery systems, owing it to their nano size, low immunogenicity, and targeting efficiency. Moreover, the functional lipid membrane and hydrophilic core of these vesicles provide a stable environment for the nanomaterials, preventing immune responses and granting fusion with the plasma membrane for direct entry into the target cell.
The initial steps in the general upstream bioprocess of producing viral vectors is the (1) retrieval and thawing of vector producer cells (VPC), followed by the (2) cell expansion in a bioreactor. The HEK293 cells, which are adherent in nature, are among the widely used cell lines in laboratory scale for vector production. However, upscaling the production poses several challenges such as the conventional scale-out approaches that make the overall process laborious, costly, and timeconsuming. Presently, bioreactors that operate with the Tide Motion principle, such as CelCradle X® and TideXcell®, cater to adherent cell lines for both lab and pilot scale production respectively, providing the optimum conditions for the cells, and altogether reducing the risks of contamination that may occur with conventional approaches. Subsequently, the cells are (3) harvested manually or through semi, or fully automated harvesting systems. For pilot scale production, the cells are expanded in larger scale Tide Motion bioreactors.
The downstream process of viral vector production involves a series of purification and filtration steps to remove impurities, both product- and process-related. (1) Clarification is performed to eliminate cell debris and cell contaminants, within the supernatant, that may cause disturbance to the downstream product quality. The clarified harvests are then subjected to (2) chromatography for further purification and removal of unwanted impurities, or adventitious agents, that are unintentionally introduced into the production process. (3) Tangential flow filtration (TFF) concentrates the viral vectors to separate and purify the sample solution by filtering the permeate and recycling the retentate back to the feed. Followed by (4) diafiltration and (5) sterile filtration for further separation and purification. The following downstream steps, formulation and filling, labelling and packaging, and storage are accomplished to establish the finished product for the sales and distribution.
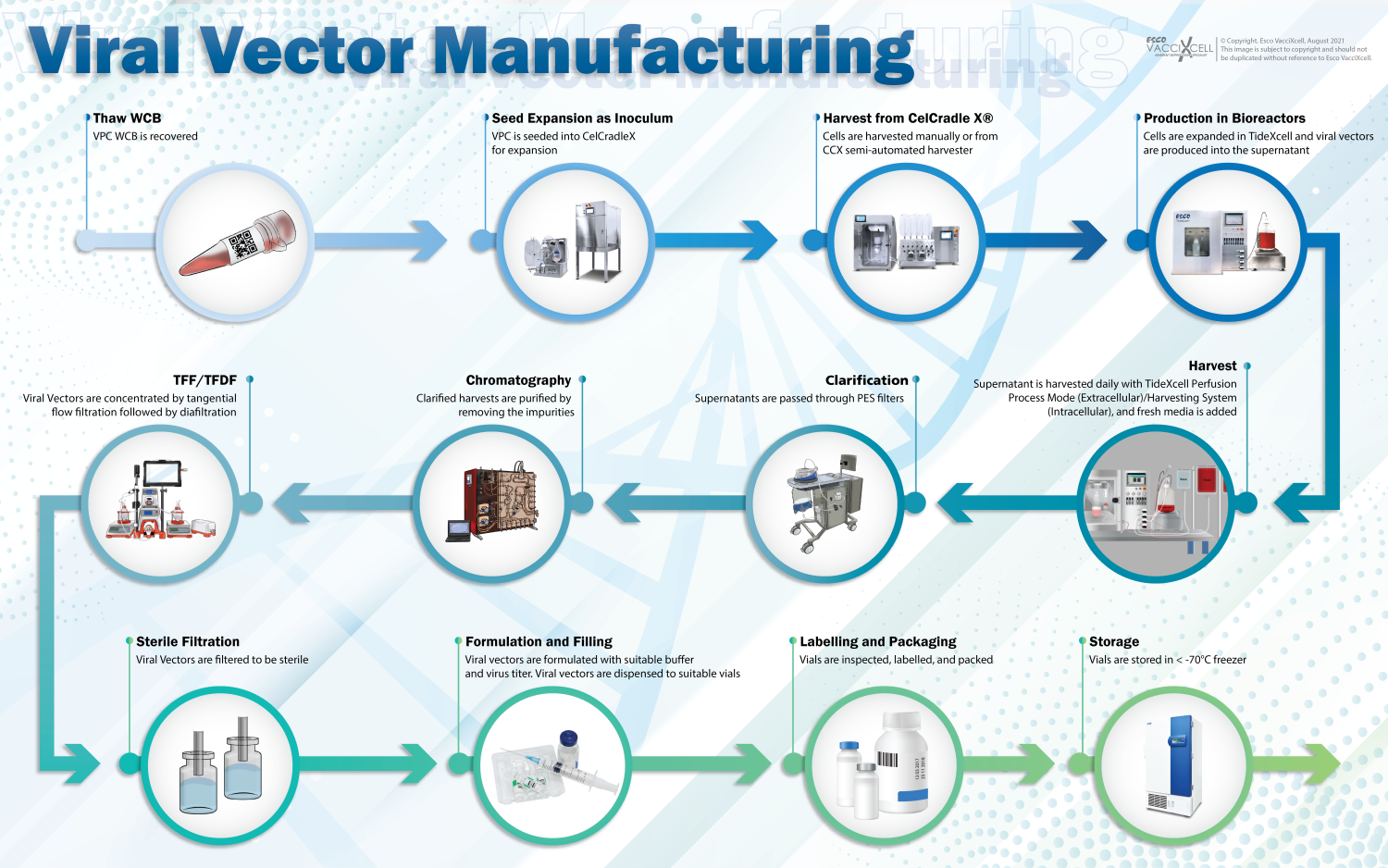
Figure 2. Manufacturing process of viral vectors for gene therapy.
As medical and pharmaceutical industries shed light on the continuous development of gene therapy, there are still arising challenges and limitations to take into account. Although different industries have accelerated the progress on gene therapy, they must continue to further the efficiencies and improve production processes for its economic viability at scale.
Depending on the vector for gene therapy, scaling out or scaling up will pose challenges and limitations. For example, autologous gene therapy requires only a single batch per patient, hence the initial option would be scaling out. This scale-out scenario is costly and time-consuming as it requires additional materials, and therefore requiring multiple skilled operators. These can be considered limiting factors in increasing capacity through scaling out.
Scaling up, on the other hand, offers more advantages as increasing the capacity of a bioreactor could produce greater volumes without additional resources. Tide Motion bioreactors such as CelCradle X® and TideXcell® could increase production through the scale-up scenario, and at the same time provide the optimal conditions for the cells. CelCradle X®, a single-use bioreactor, cuts the downtime in the upstream process that involves sample and bioreactor preparations. It is also considered simpler at laboratory scale, and safer as the chances of contamination with adventitious agents from one batch to another is relatively low in single-use bioreactors. Furthermore, the ideal bioreactor for single-use and multiple-use technology for adherent cell expansion, at pilot scale, is TideXcell®. This Tide Motion bioreactor integrates the desirable, cutting-edge features that allow monitoring and control over the conditions of the cells, providing them the most favorable environment.

CelCradle X® (Closed Automated Single-Use Bioreactor)

TideXcell® (Pilot Scale Tide Motion Bioreactor)
The risk of biocontamination can compromise the outcome of gene therapy, not only affecting the patient, but also leaving grave financial consequences, and a negative impact on the overall operation. Essentially, in gene therapy, cells should be free of adventitious agents, yet the risk of contamination is especially high throughout the manufacturing process which generally involves a series of stages that need the highest requirements of quality control to ensure Good Manufacturing Practice (GMP) compliance.
The design of closed system bioreactors provides physical barriers to avoid the exposure of cells to contaminants. In accordance with GMP compliance for strict aseptic techniques, CelCradle X® and TideXcell® for laboratory scale and pilot scale, respectively, operate in a closed system that contain the cells under controlled sterile conditions. Furthermore, these bioreactors are equipped with decontamination systems for more sterile measures.
Sign up to our newsletter and receive the latest news and updates about our products!
