- We are knowledgeable on cGMP cell culture, virus production and biologics production. Our staff have basic cell culture knowledge with proper training to support sales activities.
- We provide quick and good after-service and maintain engagement with our clients.
- We actively participate in exhibitions to demonstrate our products and find the new leads.
- The probe must be washed thoroughly with appropriate cleaning agents before and after use.
- Do not leave the end of the probe dry and keep it submerged in the electrolyte (e.g. 3 M KCl) when not in use to extend the lifespan. The electrolyte should be changed regularly.
- Be careful not to introduce any bubbles to the electrode as this will make unstable measurements.
- The pH probe can usually tolerate 20 - 50 autoclave cycles.
- The probe must be washed thoroughly with appropriate cleaning agents (e.g. ddH2O). Do not use alcohol as this can damage the sensor or lead to erroneous current.
- The membrane foil should be cleaned and properly installed prior to calibration.
- If you find fluctuation in the readings, inability to do calibration, or low DO probe current, the membrane body might need to be replaced.
- For short term storage, keep the sensor submerged in O2 electrolyte. For long term storage (at least 6 months), the probe should be stored dry.
For virus production process, we usually decide the end point by checking the glucose consumption rate, since CPE provides a rough evidence, while the glucose consumption rate is a more accurate determinant of harvesting time. Moreover, the end-point also depends on the stability of the virus during post-infection period.
- Healthy cells
- Infectious active virus
- Post-Infection temperature
- Multiplicity of Infection (MOI)
- Harvest strategy
The mechanism of CelCradle® enables fast and complete diffusion of nutrients and oxygen in the medium to the matrix. All ingredients in the medium are properly mixed while the CO2 produced by glucose hydrolysis can be expelled efficiently along the run. The CelCradle® system also has a high productivity – 1 CelCradle® bottle is equivalent to 20 x 850cm2 roller bottles, 4 x 500ml Spinner Flask, 100 x 180cm2 petri dishes, 2 x 6320cm2 plates or 125 x 150cm2 T-flasks. It also provides cells with an environment of low shear stress, zero foaming and bubbling, and no oxygen limitation.
- To use enzyme other than trypsin, for example, Accutase. Some other enzyme but not trypsin-based may also be helpful.
- To disrupt the cells from the carriers through freeze-and-thaw, with lysis buffer, or other methods.
CelCradle-500 is used mostly in the lab for research and tissue engineering whereas TideXcell-002 can be applied in both academy and small pilot tests. The final cell densities in both systems are quite related proportionally. However, the productivity and cell growth rate depend on several factors.
Since CelCradle-500 doesn’t have pH control and perfusion culture mode, the cell growth usually is slower than in TideXcell-002, under a manual operation. With respect to the linearity of scale-up, TideXcell-002 is better than CelCradle-500. The parameter setting in CelCradle-500 could be used in TideXcell-002, but some modifications are still necessary. Compared with other commercially-available systems, however, very few parameters are required to input in the TideXcell system. Therefore optimization is quite straight -forward.
CelCradle-500 is used mostly in the lab for research and tissue engineering whereas TideXcell-002 can be applied in both academy and small pilot tests. The final cell densities in both systems are quite related proportionally. However, the productivity and cell growth rate depend on several factors.
Since CelCradle-500 doesn’t have pH control and perfusion culture mode, the cell growth usualy is slower than in TideXcell-002, under a manual operation. With respect to the linearity of scale-up, TideXcell-002 is better than CelCradle-500. The parameter setting in CelCradle-500 could be used in commercially-available systems, however, very few parameters are required to input in the TideXcell System. Therefore optimization is quite straight-forward.
CelCradle is composed of CelCradle Stage, Controller, CelFeeder pump –depends on the application- and CelCradle Bottle.
CelCradle bottle is composed of 4 parts: Vented bottle Lid, Culture Container, BioNOC II® (Carrier) Container, Media Container. Depends on Bottle type, some also comprise (500A and 500AP bottles) non-vented bottle lid.
If CelCradle-500/500A is used, then just to replace culture medium manually by opening the cap inside biosafety cabinet and discard the used culture medium and then fill in fresh culture medium.
If CelCradle-500P/500AP is used, then need to replenish the culture medium in the reservoir depends on how quick the glucose is consumed.
Yes. CelCradle-500/500P equips a sampling port on the carrier basket. With sterilized forceps, users take out carriers during culture.
CelCradle-500A/500AP doesn’t have carrier basket, so with sterilized forceps, users can take out carriers, or harvest carriers.
There are two methods for users to observe the culture performance.
Way 1 : to measure the metabolite consumption in the cultured media. The pH and glucose are the bassic parameters to be monitored during culture.
Way 2 : to count cells. You can pick up carriers during culture, and count the cells by using dye.
There are two ways to solve the issue:
Way 1 : To use enzyme other than trypsin, for example, Accutase. Some other enzyme but not trypsin-based may also be helpful.
Way 2 : To disrupt the cells from the carriers through freeze-and-thaw, with lysis buffer, or other methods.
CelCradle is composed of CelCradle Stage, Controller, CelFeeder pump –depends on the application- and CelCradle Bottle.
CelCradle bottle is composed of 4 parts: Vented bottle Lid, Culture Container, BioNOC II® (Carrier) Container, Media Container. Depends on Bottle type, some also comprise (500A and 500AP bottles) non-vented bottle lid.
If CelCradle-500/500A is used, then just to replace culture medium manually by opening the cap inside biosafety cabinet and discard the used culture medium and then fill in fresh culture medium.
If CelCradle-500P/500AP is used, then need to replenish the culture medium in the reservoir depends on how quick the glucose is consumed.
Yes. CelCradle-500/500P equips a sampling port on the carrier basket. With sterilized forceps, users take out carriers during culture.
CelCradle-500A/500AP doesn’t have carrier basket, so with sterilized forceps, users can take out carriers, or harvest carriers.
There are two methods for users to observe the culture performance.
Way 1 : to measure the metabolite consumption in the cultured media. The pH and glucose are the bassic parameters to be monitored during culture.
Way 2 : to count cells. You can pick up carriers during culture, and count the cells by using dye.
There are two ways to solve the issue:
Way 1 : To use enzyme other than trypsin, for example, Accutase. Some other enzyme but not trypsin-based may also be helpful.
Way 2 : To disrupt the cells from the carriers through freeze-and-thaw, with lysis buffer, or other methods.
| Model | Vol. of BioNOC II® | Matrix volume | Equivalence to Stirred Tank Bioreactors | |
| 1:10 Mixing Ratio | 1:20 Mixing Ratio | |||
| TideXcell-002 | 110g | 2000 ml | 20L | 40L |
| TideXcell-010 | 550g | 10,000 ml | 100L | 200L |
| TideXcell-020 | 1100g | 20,000 ml | 200L | 400L |
| TideXcell-100 | 5500g | 100,000 ml | 1000L | 2000L |
| Model | Cell Seeding(Recommended) | Expected Resulting Cell Density |
| TideXcell-002 | 2x109 cells | 2x1010 cells |
| TideXcell-010 | 1x1010 cells | 1x1011 cells |
| TideXcell-020 | 2x1010 cells | 2x1011 cells |
| TideXcell-100 | 1x1011 cells | 1x1012 cells |
Yes, besides the function of "Emergency Stop", event and alarming messages provide operators real-time monitoring of process variables and historical data. All deviations and user control actions (e.g. log-in/off, door open) are listed in the record.
An optional alarming notification is through the SCADA system or mobile communication such as cell phones via e-mail.
The validation of TideXcell system includes four major phases: DQ, IQ, OQ, and MQ. The validation may involve in inspection, functional testing, and documentation review, verifying that the system can run appropriately at the customer site.
DQ, Design Qualification, defines the functional and operational specification of an instrument or system
IQ, Installation Qualification, is the pre-installation detail that an instrument or system, its component parts and location, are fit for the purpose and satisfy the objectives or the user to carry out the intended function to expected standards.
OQ is Operation Qualification, demonstrating that an instrument or system will function according to its operational specification in the selected environment.
MQ is Maintenance Qualification, describing any maintenance required.
Cells are exposed to aeration during emerging and to nutrition during submerging and therefore vertically, cells may have different aeration and nutrition times. However, our findings show no disadvantage as long as the system can provide cells with sufficient oxygen and nutrient.
The real situation is that the cells are embedded inside the carriers and there is always a layer of culture medium absorbed the carriers. Therefore, whether the system is in aeration or nutrition phase, the cells are technically always in the liquid phase. The quantity of culture medium remaining on the carriers is 20% of the entire bed volume, which is sufficient to supply the nutrient to the cells. The tide movement is to maintain the supply of oxygen and nutrients and to remove waste, a very similar concept to roller bottles.
The commercial microcarrier system is a very difficult process. Though it is relatively easy in the small scale, scaling up to production scale is difficult and complex. The microcarrier system also incurs shear stress, foaming, and bubbling, which may all lead to cell death. Cell death not only decreases productivity but also increases downstream processing costs, due to increased cell debris.
VacciXcell’s tide motion system is simple and applies a similar principle as that of the roller bottles. Most of our customers use roller bottles and transfer to our technology directly without any problems. Those that tried to use the microcarrier system failed and eventually switched to the tide motion system.
Inoculation
- Clamp the tube between Matrix Vessel and Mixing Vessel.
- Prepare at least 3x109 cells in pre-warmed 800 ml culture medium per L matrices.
- Disconnect the air tube inside TideXcell Culture Chamber with the Matrix Vessel. Move the Matrix Vessel to Biosafety cabinet.
- Load the cells into the Matrix Vessel. Mix the cell containing culture medium together with the Matrixes by gently shaking the matrix vessel in a rotational manner by hand for 3 minutes.
- Place the Matrix Vessel back to the Culture Chamber. Reconnect the air tube with the Matrix Vessel.
- Remove the clamp on the tube between the Matrix Vessel and Mixing Vessel.
For contamination issues, it highly depends on how the customer controls the culture environment and operation. Customers seldom report contamination issues to us which we think is due to the fact that most contamination issues are due to their process and practices and is rarely due to the equipment itself. Using our single-use system would make contamination risk almost 0.
In terms of component failure, we have had some cases but it is quite rare, roughly 1% and all these cases are after warranty periods. Strictly following the maintenance schedule is very important to minimize or reduce the risk of system failure.
- CHO: CHO-S-SFM II (Gibco), CHO III A (Gibco), CHO-A-SFM (Gibco) , ExCELL 301 (JRH), HyQ PF-CHO (HyClone), HyQ-CCM 5 (HyClone), HyQ SFX-CHO (HyClone), IS-CHO-CD (IRVINE), proCHO4cdm
- HEK-293: EX-CELL 293 (JRH), Pro293a-CDM (CAMBREX), HyQ SFM4 HEK293 (HyClone)
- VERO: VP-SFM (Gibco), Plus VERO (VACCIXCELL), ICN-VERO (ICN), PEEK-1 (Biochrom),
- Sf-9: SF-900 II (Gibco), EX-CELL 420 (JRH), EX-CELL 400
GlucCell Glucose Monitoring System is designed specifically for animal cell culture in which the embedded calibration curves are made. Currently we don’t have sufficient data showing that it could be used for microorganism culture with these existing calibration curves. However, following steps can be done to validate your application:
- Perform calibration with your existed culture medium with known glucose concentration by spiking known amount of glucose. If the initial glucose concentration of the medium is not adjustable, you have to prepare the medium by yourself by adding the component one by one and making several groups with different glucose concentration that could cover your test range.
- Spin-down the culture medium to remove the solid and measure the glucose only from the supernatant.
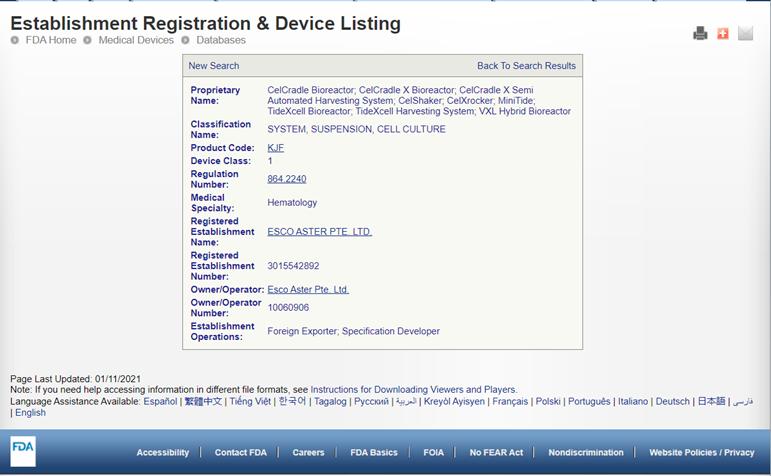
The CelCradle X® and TideXcell® systems are cGMP compliant. CelCradle, CelCradle X, CCX-SAH, CelShaker, CelXrocker, MinTide, TideXcell, TXLHS, and VXL Hybrid are ALL FDA-Listed products.
cGMP (FDA cGMP standard): CelCradle® can comply to cGMP but it relies on the clinical sponsor or the appointed CMO through manual recording as part of their Process Qualification (PQ) and quality management release. The CMO or the clinical sponsor defines their own lot release for e.g. Esco Aster have a long list of quality control and quality assurance test, including cell-based assays and downstream purification methods to purify the product from our bioreactors. Used materials in all steps are fully traceable and uses serum/xeno-free chemically defined GMP media and cryopreservation.
CelCradle® can be GMP-compliant if it is applied under cGMP (FDA cGMP standard) provided that they use a cGMP CO2 incubator that is connected to a Scada or Dcs system with eBatch records and eSignature.
If you are not using a cGMP incubator, manual logging is needed as part of Quality Management system and SOP or protocol. They should manually record in their production records what was adjusted in the incubator or cell manipulation, which person and at which time. This should also indicate which supervisor verified and who did the process QC to manually sample the BioNOC II during culture.
Disclaimer: cGMP/GMP (Iso 13485) may differ depending on your local authority who is final regulatory body, these suggestions from Esco as equipment manufacturer should not be mis used or mis represented. Your country local authority has final say in all cGMP/GMP matters.