TideXcell ® Pilot Scale Bioreactor
The Gentle Giant of Adherent Bioprocessing






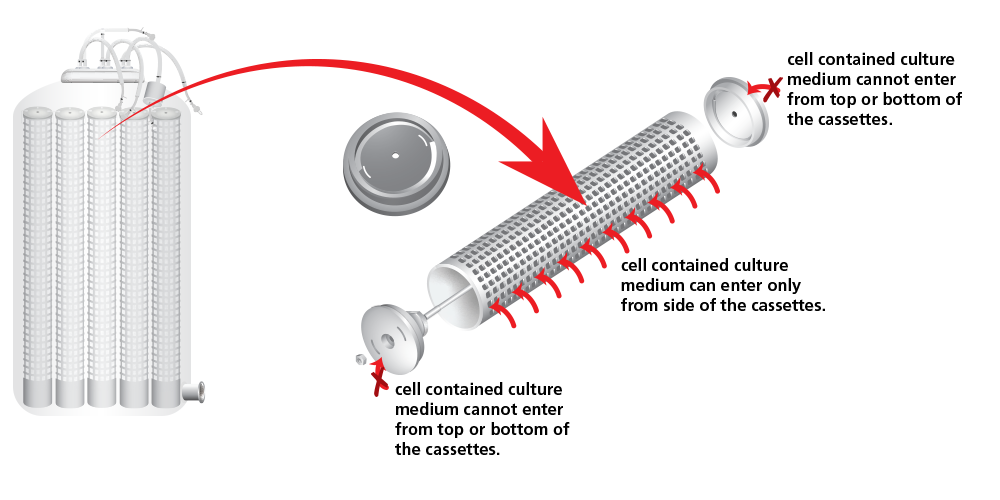
TideXcell ® Matrix Bed is designed to cut the area into numerous small sections and force cells to flow in horizontal radial direction instead of vertical direction. The gradient effect during the seeding phase could be minimized.
A matrix cassette is packed with carriers configured in 3D for optimum cell growth. Cassettes are stacked inside the matrix vessel(2 L, 20 L, 100L) for uniform seeding of cells.

TideXcell ® Matrix Bed is designed to cut the area into numerous small sections and force cells to flow in horizontal radial direction instead of vertical direction. The gradient effect during the seeding phase could be minimized.
The concept is achieved by packing the matrix bed with porous cassettes with smaller diameter when matrixes are packed inside.
Continuous harvesting can be done in the TideXcell system due to 100% media exchange. This process is beneficial when doing viral culture work for producing higher viral titers or for stem cell culture which requires switching to a different media for differentiation.
Due to the seperation of the matrix vessel (where cells reside) and the mixing vessel (where the mixing of the culture medium is happening), the system is capable of fully harvesting the waste or conditioned media and feeding in a 100% fresh culture medium afterwards without harming the cells being cultured.
Closed system sampling can be done in the TideXcell ® system. Each vessel types have their own corresponding number of sampling port.

A matrix cassette is packed with carriers configured in 3D for optimum cell growth. Cassettes are stacked inside the matrix vessel (2 L, 20 L, 100 L) for uniform seeding of cells.
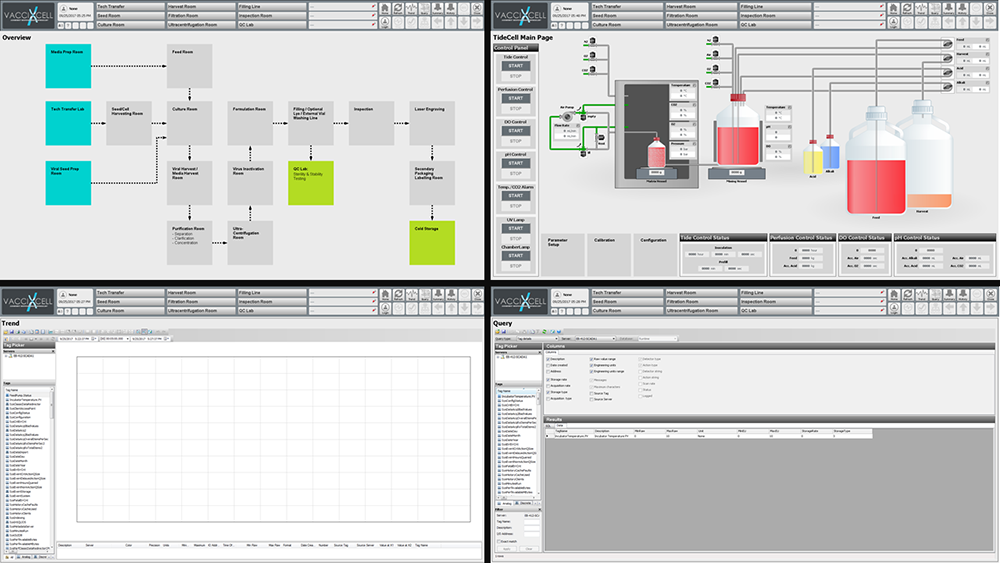
TideXcell ® PLC-based monitoring and control structure runs on WonderWare SCADA. This provides a high-level process supervisory management and data acquisition. This software platform will ensure process control safety, will support control strategy, and will provide a remote method of capturing data and events (alarms) for monitoring the continuous process. SCADA platforms also provide functions for graphical displays, alarms, trends and historical storage of data.
TideXcell ® is the core of Esco Aster, a contract development manufacturing organization (CDMO) that uses single-use adherent bioreactors in bioprocessing needs. Esco Aster focuses on process development, commercialization of new translational drugs (NTDs) for both humans and animals, orphan drugs, as a CMO for partner TideXcell ® factories looking to gain access in the ASEAN, ANZ, African region.


Cells are exposed to aeration during emerging and to nutrition during submerging and therefore vertically, cells may have different aeration and nutrition times. However, our findings show no disadvantage as long as the system can provide cells with sufficient oxygen and nutrients.
The real situation is that the cells are embedded inside the carriers and there is always a layer of culture medium absorbed by the carriers. Therefore, whether the system is in the aeration or nutrition phase, the cells are technically always in the liquid phase. The quantity of culture medium remaining on the carriers is 20% of the entire bed volume, which is sufficient to supply the nutrient to the cells. The tide movement is to maintain the supply of oxygen and nutrients and to remove waste, a very similar concept to roller bottles.
| Model | Vol. of BioNOC™ II | Matrix volume | Equivalence to Stirred Tank Bioreactors | |
|---|---|---|---|---|
| 1:10 Mixing Ratio | 1:20 Mixing Ratio | |||
| TideXcell-002 | 110g | 2000 ml | 20L | 40L |
| TideXcell-010 | 550g | 10,000 ml | 100L | 200L |
| TideXcell-020 | 1100g | 20,000 ml | 200L | 400L |
| TideXcell-100 | 5500g | 100,000 ml | 1000L | 2000L |
| Model | Cell Seeding (Recommended) | Expected Resulting Cell Density |
|---|---|---|
| TideXcell-002 | 2x10 9 cells | 2x10 10 cells |
| TideXcell-010 | 1x10 10 cells | 1x10 11 cells |
| TideXcell-020 | 2x10 10 cells | 2x10 11 cells |
| TideXcell-100 | 1x10 11 cells | 1x10 12 cells |
Yes, besides the function of "Emergency Stop", event and alarming messages provide operators real-time monitoring of process variables and historical data. All deviations and user control actions (e.g. log-in/off, door open) are listed in the record.
An optional alarming notification is through the SCADA system or mobile communication such as cell phones via e-mail.
The validation of TideXcell system includes four major phases: DQ, IQ, OQ, and MQ. The validation may involve inspection, functional testing, and documentation review, verifying that the system can run appropriately at the customer site.
DQ, Design Qualification, defines the functional and operational specification of an instrument or system.
IQ, Installation Qualification, is the pre-installation detail that an instrument or system, its component parts and location, are fit for the purpose and satisfy the objectives or the user to carry out the intended function to expected standards.
OQ is Operation Qualification, demonstrating that an instrument or system will function according to its operational specification in the selected environment.
MQ is Maintenance Qualification, describing any maintenance required.
The commercial microcarrier system is a very difficult process. Though it is relatively easy in the small scale, scaling up to production scale is difficult and complex. The microcarrier system also incurs shear stress, foaming, and bubbling, which may all lead to cell death. Cell death not only decreases productivity but also increases downstream processing costs, due to increased cell debris.
Esco Healthcare’s tide motion system is simple and applies a similar principle as that of the roller bottles. Most of our customers use roller bottles and transfer to our technology directly without any problems. Those that tried to use the microcarrier system failed and eventually switched to the tide motion system.
For contamination issues, it highly depends on how the customer controls the culture environment and operation. Customers seldom report contamination issues to us which we think is due to the fact that most contamination issues are due to their process and practices and is rarely due to the equipment itself. Using our single-use system would make contamination risk almost 0.
In terms of component failure, we have had some cases but it is quite rare, roughly 1% and all these cases are after warranty periods. Strictly following the maintenance schedule is very important to minimize or reduce the risk of system failure.
| Construction | External Carcass: 316 stainless steel | |
| Dimension | 125 (W) X 70 (D) X 115 (H) cm | |
| Weight | 240 kg | |
| Electrical requirements | AC 220V 20A, 50/60 Hz, Single phase UPS (uninterrupted power supply) width 6kVA/4.2kW should be prepared by customers |
|
| Control Hardware (2-20L) | Flexible PC-based/DAQ industrial control interface; Solenoid valves/PID gauges/Pressure-vacuum motor NDIR CO 2 diffusion type sensor |
|
| Control Hardware (50-100 L) | Siemens HMI/PLC based control structure Solenoid valves/PID gauges/Pressure-vacuum motor NDIR CO 2 diffusion type sensor |
|
| Control Software | Siemens PLC-based control and monitoring structure Siemens HMI with 12.1" touchscreen Development environment: TIA Protal V13 SP1 |
|
| Incubator | 0 - 20% CO2 PID Control +8ºC~27ºC Operating Temperature Front view window; LED inside lighting Emergency power-off button |
|
| Connection | CPC nickel-coated brass quick connectors Electric connectors with locking-screw |
|
| Communication | 9-pin Dsub RS-485 port : Modbus RTU protocol 2 USB Ports for import firmware / software upgrade and export trend data |
|
| Construction | 264 W x 359 L X 170 H mm (10.4 x 14 x 6.7 inches) | |
| Control Features | Simple user's administration Process page & Date viewer page Data logging/Parameters logging/Events logging Individual seed and amp Cultivation conditions setting with default values Automatic switch from seeding stage to cultivation stage up to 300 minutes Automatic air refreshing mechanism |
|
| Tidal control | 800 ~ 1, 800 mL/min | |
| Gas flow rate | 0.8 – 20 LPM (depends on system size) | |
| Alkaline addition | 80 mL/min at 100 rpm | |
| Perfusion rate | 80 mL/min at 100 rpm | |
| File export | Excel (.xlx) | |
| pH control | 4~10 ± 0.1 | |
| DO control | 0%~100% ± 0.5% | |
| CO 2 control | 0%~10% ± 0.3% | |
| Protection | Over pressure protection (max. 1 bar) Liquid leaking protection Over suction protection Over time protection |
|
| Magnetic Stirrer Mixing System | Recirculation Thermostatic Mixing System | Stir-tank Mixing System | |
| Construction | Magnetic stirrer 10 L, 20 L borosilicate glass vessel with stir bar for mixing |
Recirculation Mixing with magnetic driven bearing-less impeller |
Stir mixing with pre-installed impeller |
| Electrical Requirements | Monophase 3-threads AC 220V ± 10V 5A max 50/60 Hz |
Single phase, AC 220v, 9A max, 50/60 Hz |
Three phase, 230 VAC, 50/60 Hz |
| Capacity | 10 L and 20 L | 50 L, 100 L 200 L, 500 L, 1,000 L | 30 L, 50 L, 100 L, 200 L, 500 L, 1,000 L |
| Control | 100 – 1,500 rpm stir rate RT to +45°C temperature control |
Pumping rate 0 – 21 LPM RT to +50ºC temperature control |
80 – 1,000 rpm stir rate RT to +60ºC temperature control SIP system (sterilization in place) |
The following table shows cGMP compliant customers using TideCell in their projects. Company identities and some other information are hidden due to Non-disclosure agreement (NDA) between the company and VacciXcell
| SECRETED VIRUS | ||||||
|
Country |
Cell Line |
Virus Strain |
Cell Density |
Virus Titer |
cGMP |
SCADA/DCS |
|
Taiwan |
VERO |
Japanese Encephalitis virus |
4.6 x107 |
109pfu/ml |
Yes |
- |
|
China |
VERO |
Rabies virus |
4.0 x107 |
108 IgLD50/ml |
Yes |
- |
|
Taiwan |
VERO |
Enterovirus type 71 |
2.6 x107 |
107.8 TCID50/ml |
Yes |
- |
|
Taiwan |
MDCK |
H5N1 |
2.3 x107 |
HA=1024~2048 |
Yes |
SCADA |
|
Taiwan |
MDCK |
A(H1N1) |
2.2 x107 |
HA=512~1024 |
Yes |
- |
|
Taiwan |
PK |
Hog Cholera |
3.7 x107 |
106.5pfu/ml |
Yes |
- |
|
China |
ST |
Hog Cholera |
2 x107 |
2 mil. RID/ml |
Yes |
- |
|
China |
BHK-21 |
Rabies |
4.5x107 |
108.5 TCID50/ml |
Yes |
- |
|
Japan |
MDBK |
(NDA) virus for Bovine vaccine |
1.29x107 |
109.2 TCID50/ml |
Yes |
|
|
China |
Marc145 |
PRRSV |
1.16x107 |
108.29 TCID50/mL |
Yes |
- |
|
Spain |
NDA |
(NDA) Virus of Bovine Vaccine |
2.6 × 107 |
N/A |
Yes |
- |
| NON-SECRETED VIRUS | ||||||
|
Country |
Cell Line |
Virus Strain |
Cell Density |
Virus Titer |
cGMP |
SCADA/DCS |
|
Japan |
NDA |
Hepatitis A |
2×1011 |
N/A |
Yes |
SCADA |
Sign up to our newsletter and receive the latest news and updates about our products!
