Applications
Inorganic Vectors: The Next Generation Delivery System in Gene Therapy
An inorganic viral vector refers to a type of gene delivery system that uses inorganic materials, such as nanoparticles, to transport genetic material into cells. In contrast to organic viral vectors, which use modified viruses to carry genetic material, inorganic viral vectors offer several advantages, including their ability to overcome some of the limitations associated with traditional gene therapy approaches.
Inorganic vectors can be made from a variety of materials, including gold, silica, and iron oxide nanoparticles. They are designed to be biocompatible and non-toxic to cells, while also being highly efficient at delivering genetic material into cells.
Inorganic vectors have been used in a variety of applications, including gene therapy, cancer treatment, and vaccine development. They offer several advantages over traditional gene therapy approaches, such as improved safety, reduced immune response, and greater control over gene expression.
Types of Inorganic Vectors
There are various types of inorganic vectors that are being developed for gene delivery applications. Some of the most common types include:
Gold nanoparticles: Gold nanoparticles are a popular choice for inorganic viral vectors due to their biocompatibility and ease of functionalization. They can be modified with a variety of molecules, such as antibodies, peptides, or DNA, to facilitate targeted delivery to specific cell types.
Silica nanoparticles: Silica nanoparticles are another commonly used inorganic viral vector. They offer several advantages, including high stability, ease of functionalization, and low toxicity.
Iron oxide nanoparticles: Iron oxide nanoparticles are often used for magnetic targeting and imaging applications. They can be used with targeting ligands to enable selective delivery to specific cells or tissues.
Calcium phosphate nanoparticles: Calcium phosphate nanoparticles are biocompatible and biodegradable, making them an attractive option for gene delivery. They can be functionalized with a variety of molecules, such as DNA, RNA, or proteins, to enable efficient gene delivery to cells.
Carbon nanotubes: Carbon nanotubes are an emerging class of inorganic viral vectors that offer several advantages, including high stability, ease of functionalization, and the ability to penetrate cell membranes.
Advantages of Inorganic Viral Vectors
Inorganic viral vectors offer several advantages over traditional gene therapy approaches, making them an important and promising tool for gene delivery.
Here are some of the key advantages and importance of inorganic viral vectors:
- High efficiency: Inorganic viral vectors are shown to be highly efficient at delivering genetic material into cells, resulting in high levels of gene expression. This is particularly important for therapeutic applications, where high levels of gene expression are necessary to achieve a therapeutic effect.
- Improved safety: Inorganic viral vectors are generally considered to be safer than organic viral vectors, which can cause immune reactions and have potential for unintended integration into the genome. Inorganic viral vectors can be engineered to be biocompatible and non-toxic to cells, reducing the risk of adverse effects.
- Reduced immune response: Inorganic viral vectors can be engineered to be stealthy and evade the immune system, reducing the risk of immune reactions and allowing for repeated administration.
- Greater control over gene expression: Inorganic viral vectors can be engineered to have greater control over gene expression, allowing for more precise regulation of gene expression in target cells.
- Versatility: Inorganic viral vectors can be engineered to target a wide range of cell types and tissues, making them a versatile tool for gene delivery in a variety of applications, such as gene therapy, cancer treatment, and vaccine development.
Manufacturing Inorganic Vector for Gene Therapy
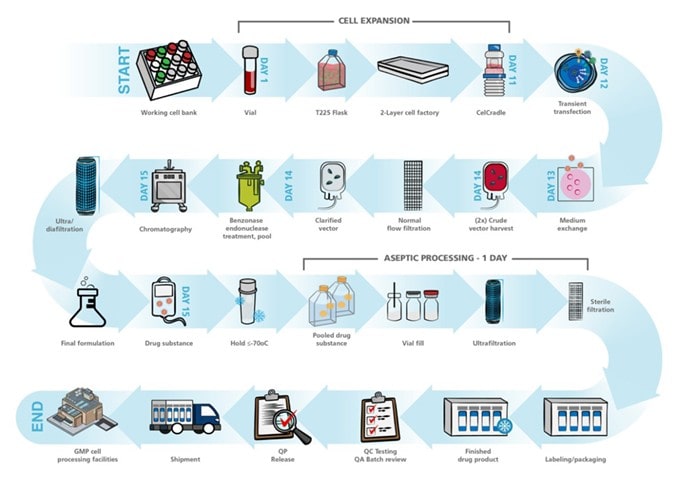
The workflow for vector production depends on the type of vector being produced, the host cells used, and the specific requirements of the therapeutic application. The typical workflow for viral vector production involves the following steps:
- Design and construction of the vector: The first step is to design and construct the vector by inserting the therapeutic gene into the viral genome using recombinant DNA technology. The vector should be designed to ensure efficient gene transfer and expression in target cells, while minimizing the risk of toxicity or immune responses.
- Transfection of host cells: The vector is then introduced into host cells, which are typically cultured in bioreactors. The cells used as host cells depend on the type of vector, and may include HEK293, CHO, or insect cells. The vector is transfected into the host cells using methods such as electroporation or lipofection.
- Expansion of host cells: The transfected host cells are then grown in Tide Motion bioreactor to allow for vector replication and production. The cells are usually grown in serum-free media, and the culture conditions are optimized to ensure high cell viability and vector yield.
- Harvesting and purification of vectors: Once the host cells have produced sufficient quantities of the vector, the culture medium is harvested, and the vector particles are purified using a combination of filtration, chromatography, and ultracentrifugation. Purification is important to remove contaminants and ensure that the vector is safe for use in humans.
- Quality control: Quality control measures are applied throughout the vector production process to ensure that the final product meets the required specifications for safety and efficacy. This includes testing for sterility, identity, potency, and purity.
- Formulation and packaging: Once the vector has been purified and characterized, it is formulated and packaged for delivery to the patient. The vector may be formulated with stabilizers, preservatives, or other excipients to ensure stability during storage and delivery.
- Administration to the patient: The final step is to administer the vector to the patient either through direct injection or ex vivo gene therapy. The vector delivers the therapeutic gene to the target cells, where it can correct or replace defective or missing genes.
Limitations and Challenges
Although inorganic vectors have many potential advantages in gene therapy, they also have some limitations and challenges that need to be addressed.
One major limitation of inorganic vectors is their potential toxicity. Some inorganic materials, such as certain types of metal nanoparticles, can be toxic to cells and tissues, causing inflammation, oxidative stress, and other harmful effects. The toxicity of inorganic vectors can also depend on their size, shape, surface charge, and other physicochemical properties, which can affect their interactions with cells and biological systems.
Another limitation of inorganic vectors is their potential for non-specific binding and uptake by cells. Inorganic vectors may interact with a wide range of cells and tissues in the body, leading to off-target effects and reducing the efficiency of gene delivery to the target cells. This can also increase the risk of toxicity and other adverse effects.
Finally, the long-term fate and degradation of inorganic vectors in the body is not yet fully understood. Some inorganic materials may accumulate in tissues or organs over time, leading to potential long-term effects on the body.
Overall, while inorganic vectors have many potential advantages in gene therapy, their limitations and challenges need to be carefully considered and addressed to ensure their safety and efficacy. Further research is needed to fully understand the potential risks and benefits of different types of inorganic vectors for gene therapy applications.
References:
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771-782. doi: 10.1038/nrd2614.
- Zhao Y, Jiang Y, Lv W, et al. A review on the application of inorganic nanoparticles in gene delivery. J Mater Chem B. 2019;7(9):1449-1470. doi: 10.1039/C8TB02761C.
- Liu Y, Zhang L, Zhou C, et al. Inorganic nanoparticles for cancer therapy: a transition from lab to clinic. Nano Today. 2020;35:100944. doi: 10.1016/j.nantod.2020.100944.
- Gao J, Huang X, Liu H, et al. Metal-based nanoparticles for the diagnosis and treatment of infectious diseases. Front Bioeng Biotechnol. 2021;9:639057. doi: 10.3389/fbioe.2021.639057.





