The COVID-19 pandemic is an uncharted territory where almost all countries of the world have established the use of mouth-and-nose protection (i.e. face masks, face shield) to prevent virus transmission. As part of the comprehensive package of heath measures, everyone is advised to wash their hands, to practice social distancing, and to sanitize. The spreading rate is so high that entertainment establishments, recreational areas, and even some nations are on lockdowns which triggered global economic downturns.
Vaccines are hailed to be an exit strategy for this surging pandemic. They do not just help keep a person healthy but also greatly affect the community conclusively. If a significant number of the population is vaccinated, the spread of disease can be stopped. A recently released vaccine landscape from WHO shows what vaccines are underway to completion.
Frontrunners for producing a candidate vaccine are in Phase 3, each having different strategies on how to activate an immune response.
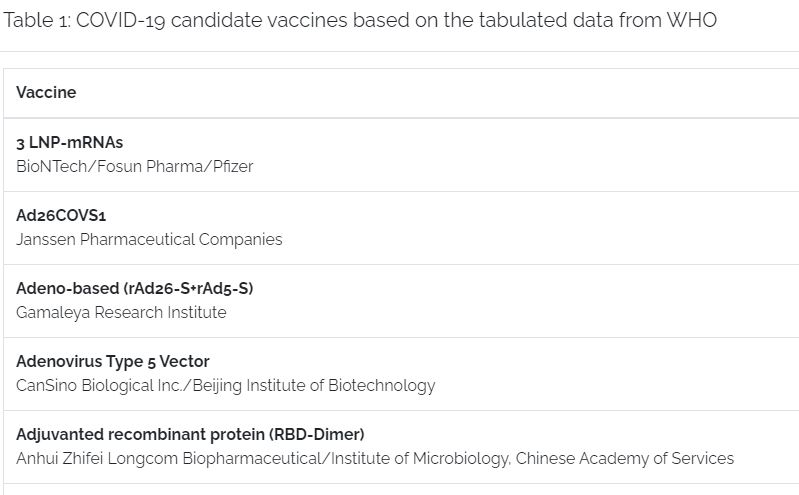
COVID-19 candidate vaccines based on the tabulated data from WHO
With COVID-19 information early release to the public, scientists pushed forward to study the model of the virus. Each of the COVID-19 vaccine mentioned have identified and used the SARS-CoV-2 spike glycoprotein, which the virus uses to infect cells, to trigger the immune system. This in turn will generate protective antibodies and a dedicated immune response to the virus once introduced to the system. The protective antibodies will act through preventing the spike glycoprotein from attaching the virus to human cells, thereby neutralizing the SARS-CoV-2 virus that causes COVID-19.
Whether the world is ready to produce billions of vaccines to accommodate the population, let us look on how vaccines are produced (Fig.1)

A general overview of the vaccine production timeline.
Vaccines take time before they are released in the market. Some have high hopes that a usable vaccine will be released by the end of this year or next year. The coronavirus vaccine landscape has about at least 180 potential candidates in the pipeline (35 candidates for clinical evaluation; 145 candidates for preclinical evaluation).
The pre-clinical stage takes 1-2 years, from the start of discovery to exploration of the targeted antigen. It requires extensive effort to make sure each step is done safely and precisely. However, if a certain vaccine is approved, testing, mass-manufacturing, and distributing these vaccines fairly and affordably will be another challenge. This landscape is divided into three types of vaccines: RNA (Fig.2), attenuated (Fig.3), and inactivated (Fig. 4).
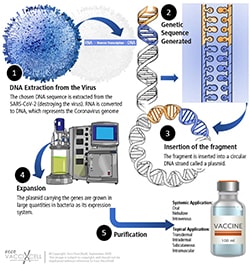
RNA vaccine workflow
RNA vaccines are faster and cheaper to make. One of its major advantages is that RNA can be produced from a DNA template in the laboratory using readily available materials. Its mechanism comprises of mRNA strand that codes for a disease-specific antigen. Once introduced into the body, the cells use the genetic information from the mRNA strand to produce the antigen; therefore, activating an immune response.
As RNA vaccines are not made with pathogenic particles or inactivated pathogen, they are deemed safe to use. In terms of production, they can be produced rapidly with faster scalability, improving emerging outbreak response.
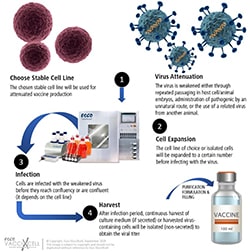
Attenuated vaccine workflow
Live, attenuated vaccines are known to be the best teachers for the immune system. The reason behind is: attenuated form of the virus is nearly identical to a natural infection. Mostly with a single dose, the recipient produces immunity against the disease. While this is true, attenuated vaccines takes longer time to make due to the manufacturing capabilities, as well as parameter optimization to obtain maximum results. One must keep an eye on how these manufactured vaccines are stored to maintain its best condition, its limitation of use, and possible reversion to virulence.
Inactivated vaccines are vaccines where the produced bacterium or virus in culture media is “killed” or inactivated with heat or chemicals such as formalin. In other cases, the antigen is purified and treated to obtain components that are to be included in the vaccine (e.g. polysaccharide).
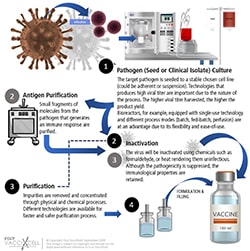
Inactivated vaccine workflow
In general, inactivated vaccines do not produce protective immunity on the first dose. It primes the immune system and will develop a protective response on the second or third dose. The response is mostly humoral as compared to attenuated vaccines: antibody titers against inactivated antigens diminish with time. In terms of production, this type of vaccine is costlier due to the duration of immunity; thus, requiring booster shots, requirement for adjuvant, and more.
Where we stand now in the course of the pandemic is changing. Although it takes 12-36 months to manufacture a vaccine alone, its promise to protect the people remains the same.
Want to know about the latest info on vaccines and COVID-19? Join us for an upcoming virtual conference and be one of the first receivers of this information.

September 28 to October 1, 2020 | 9:00 EST (GMT +4)
Esco Aster Virtual Booth
Register here
Sign up to our newsletter and receive the latest news and updates about our products!
