
Health authorities around the world are on high alert due to an outbreak of respiratory illness caused by a novel coronavirus that was first detected at the Chinese city of Wuhan. The World Health Organization has declared a coronavirus outbreak December of last year, making it an international public health emergency.
This COVID-19 outbreak, recently called the 2019 novel coronavirus (2019-nCoV), calls for vaccines. Getting vaccinated helps and trains the body's immune system to fight this disease and prevent it from spreading. With more than 85,000 confirmed cases and more than 3,000 deaths worldwide as of Mar. 2, 2020, researchers are on a race to create a vaccine with a shorter production timeline.

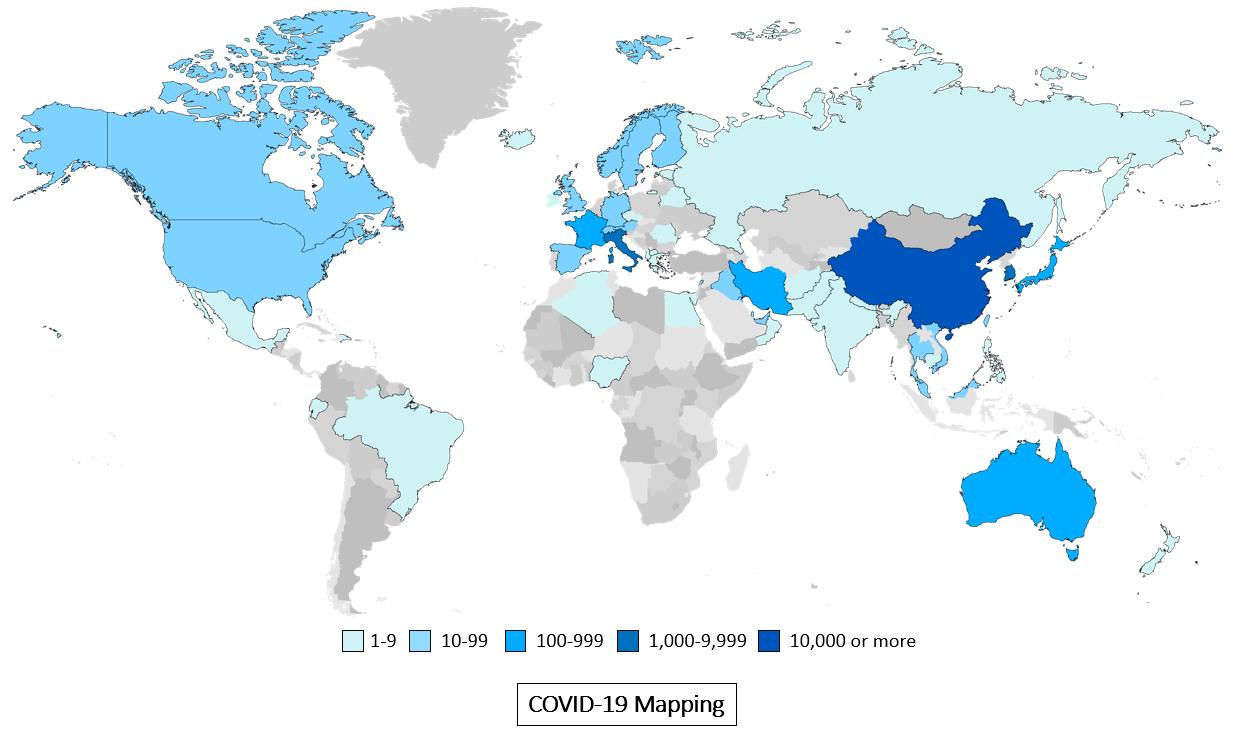

Sources: Bloomberg reporting, National Health Commission of the PRC and DXN.cn as of Mar. 2, 2020
Scientists can develop a potential vaccine with the help of the information released by Chinese officials about the virus' genetic code. With technological advances and government commitment, research facilities are able to spring into action as fast as possible. Here are some types of vaccine that have the potential to become major coronavirus vaccines to prevent the spread of the disease.
SARS-CoV-2 Vaccine Development Programs
|
Vaccine Technology |
No. of Companies Developing |
Possible Expression System |
|
Viral Vector |
8 |
Bacteria, Mammalian (adherent or suspension) |
|
DNA |
3 |
Bacteria |
|
RNA |
7 |
Bacteria |
|
Protein-based |
15 |
Yeast, Bacteria, Insect, Mammalian (adherent or suspension) |
|
Live-attenuated |
1 |
Bacteria, Mammalian (adherent or suspension) |
Source: Koch, S. (2020, February) The count of companies developing vaccines for coronavirus rises. Retrieved from https://www.biocentury.com/article/304412
DNA Vaccines
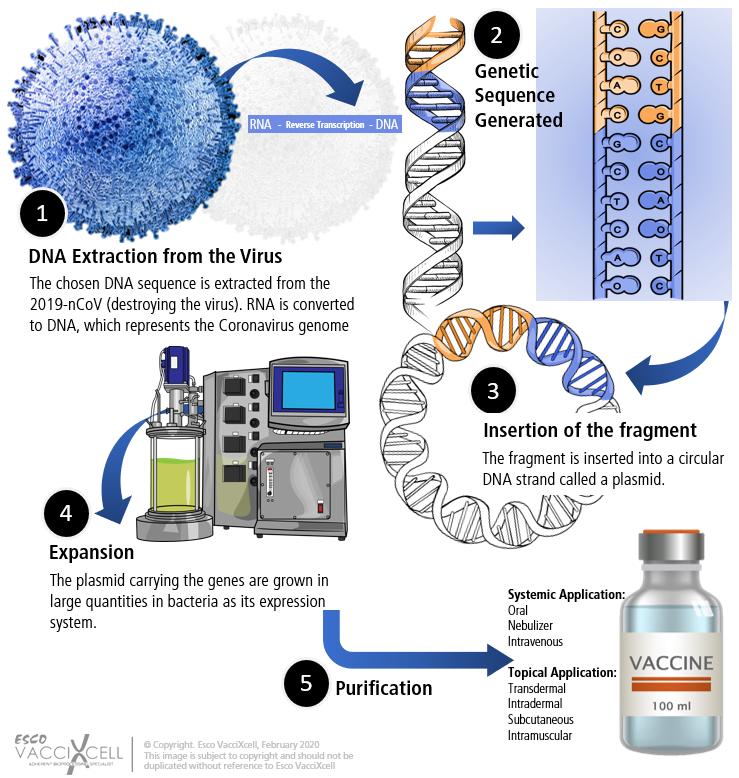
This type of vaccine provides accelerated form in developing a vaccine especially with this type of outbreak. Given the released genetic code, this vaccine technology uses a specific DNA sequence of the virus for coding a viral protein. The coded viral protein is a small piece of the virus which does not cause illness. This DNA sequence is then inserted into a genetically engineered plasmid between a strong promoter and a poly(A) signal. The plasmid can be replicated in bacterial cells and then purified for vaccine use.
Excluding Coronavirus, strong induction of immune responses to viral pathogens in animal models have been reported in DNA vaccines; however, further tests are needed when it comes to trials in human subjects. Administration can be done systemically or topically (e.g. intramuscular injection). This new DNA platform technology allows scientists to identify commonalities between two viruses and alter existing vaccines rather than building them from scratch. This greatly speeds up the process and makes the resulting drug more durable and easier to produce at a larger scale.
Manufacturing DNA Vaccines:
Naked plasmid DNA (pDNA) is obtained from desired gene-containing bacterial cells through extraction and purification. Initial vector construction and cell bank preparation takes 1-2 weeks and then transformed into a recombinant DNA. Since bacterial cells are used as an expression system, pDNA can be grown under standard fermentation conditions and manufactured in large stirred tank bioreactors. Fed-batch methods for culturing bacterial cells as it has been demonstrated to achieve high biomass, which is necessary for large-scale production. Depending on the scale, cell or product harvest for this type of vaccine can either be centrifugation or tangential flow filtration (TFF). Filtration is generally preferred at all scales due to the goal of harvesting large biomass. If the product is non-secreted, the cells are lysed and will undergo further clarification, concentration, and purification steps.
Esco VacciXcell offers stirred tank bioreactors from 5 L all the way to 2,000 L production scale. StirCradle™ combines vessel, workbench, controller, and peristaltic pumps into one integrated system. Process modes such as batch and fed-batch, which is important in manufacturing DNA vaccines, are available making it ideal for research and development. The StirCradle™ PRO is a fully automated stirred tank bioreactor for large scale expansion of bacterial cells.
Vectored Vaccines
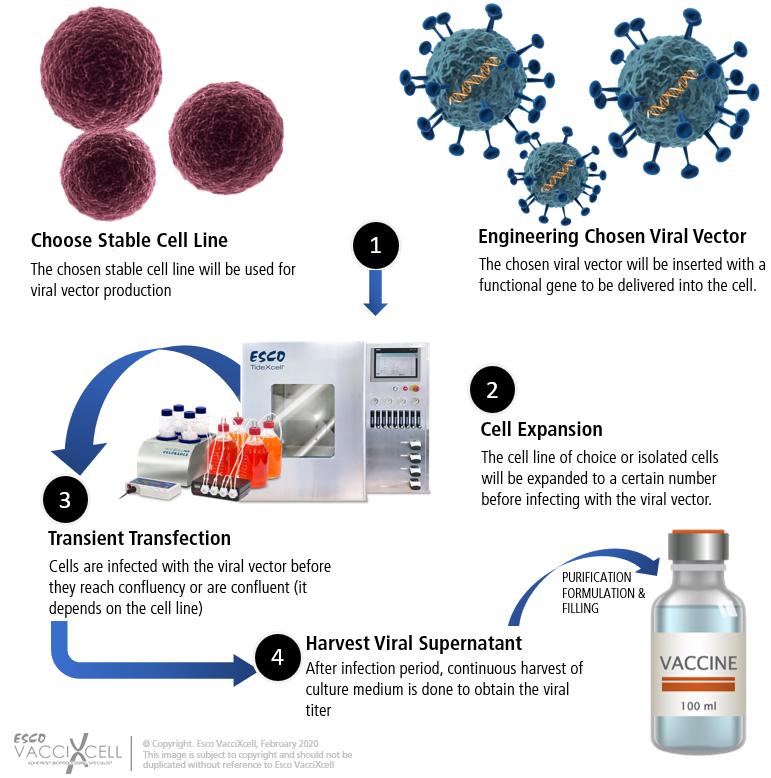
Viral vectors have demonstrated a successful approach in delivering viral DNA into a host cell for production of antigenic proteins to stimulate a range of immune response. Unlike DNA vaccines, viral vectors have high gene transduction ability, precise gene delivery to target cells, elicit optimum cellular immune response when compared to subunit vaccines where the latter elicits humoral immune response. Several groups have been utilized as a vector for the SARS-CoV structural proteins, which may also be done for other types of coronavirus. This design may be used to create a vector vaccine against the SARS-CoV-2.
Generally, viral vector vaccines contain a live attenuated virus where the disease-causing part of the virus is removed and is genetically engineered to carry DNA encoding protein antigens from an unrelated organism. Although promising, vectored vaccine production is quite slower than DNA vaccine due to the process workflow that needs to be done.
Exempted are those with BSL 3 laboratories using BSL 3 work practices, CDC does NOT recommend virus isolation in cell culture and initial characterization of viral agents recovered in cultures of SARS-CoV-2 at this time.
Manufacturing Vectored Vaccines:
Several viral vectors have been used for vaccines of different diseases and in gene therapy. Vectored vaccines are distinct wherein the properties of the vector to be used is determined by the virus from which it derives. Mammalian cells either in suspension or adherent-based are usually used as expression systems. The chosen cell line is grown in laboratory conditions and is later transfected with the virus when a certain percentage of confluency is reached. Transfecting the virus into HEK293 cell lines is a widely used platform for viral vector generation. There are commercially available systems for manufacturing these mammalian cells. Choosing the system for vectored vaccines, however, requires whether the product is harvested from the cell culture media (secreted), from cells (non-secreted), or from both.
Esco VacciXcell Tide Motion bioreactors (CelCradle™ and TideXcell™) are scalable high-density adherent cell culture production platforms, which can be used for manufacturing viral vectors. Vector production in macroporous carriers is 10 times or more efficient as compared with traditional 2D systems like cell factories. The material (BioNOC™ II ) provides enough surface area for cell growth and efficient collection of the virus from cell supernatant without having the need for cell retention devices. Although suspension cell-based cell cultures provide true scalability from small scale to large scale, not all expression systems which are adherent in nature allow adaptation to a serum-free suspension culture whilst maintaining high productivity. Varied cell densities are also noticeable in suspension cultures as compared with fixed-bed bioreactors.
Recombinant Vaccines
The main difference of recombinant vaccines to DNA vaccines is that biological constructs encoding proteins are expressed from specific viral pathogens and are not themselves made of DNA. Instead, the recombinant protein is made of protein or glycoprotein subunits synthesized in the laboratory. It relies on the capacity of one of multiple antigens using a variety of expression systems such as bacteria, yeast, insect, plant, mammalian, and cell free, to produce large quantities of the recombinant protein. The recombinant protein expressed by plasmids or harmless bacterial/viral vectors to induce immune response. Bacterial expression is the most popular expression system for recombinant proteins, however for those viruses that have post-translational modifications, mammalian expression systems work best.
One company is working on developing the vaccine based on the trimeric S protein (S-Trimer) of the SARS-CoV-2, which is responsible for binding with the host cell and causing a viral infection. cGMP biomanufacturing capabilities are needed should this type of vaccine be proven successful and be mass produced. Mammalian cell-culture based expression system is also used for the virus for better recombinant protein secretion.
Manufacturing Recombinant Vaccines:
The possibility of protective immunity induction without side effects is one of the main reasons why recombinant vaccines are being developed. A well-established technique where a heterologous host is used for protein production may be used, as well as live delivery systems (viral or bacterial vaccine delivery), or nucleic acids. The expression system for this type of vaccine manufacturing depends on the following factors: 1) post translational modifications (PTMs) requirements, 2) target protein proteolytic stability, 3) secreted or non-secreted product, 4) production costing, 5) renaturation possibility of produced misfolded protein. Given the factors, four (4) major expression systems can be used; bacterial, yeast, insect cell lines, and mammalian expression systems. Plant expression systems have also emerged but are still in development.
E. coli, a commonly used bacterial cell for expressing vaccine antigens, is well-defined and a high yielding host system. This bacterium can be produced in large colonies in stirred tank bioreactors like StirCradle™ and StirCradle™ PRO. As bacteria are easily manipulated and cultured, they are also advantageous for those types of recombinant vaccine that does not require significant post-translational modifications.
On the other hand, one of the best examples for recombinant protein vaccine, Hepatitis B virus (HBV) vaccine has been developed and approved for human use. The HB surface antigen is expressed in yeast cells and this antigen is secreted into the medium which makes it possible for continuous harvest of the supernatant. Another example would be the recently developed human papilloma virus (HPV) vaccine. The vaccine utilized the L1 recombinant proteins of each virus subtype and are produced in large amount in either yeast or insect cell system.
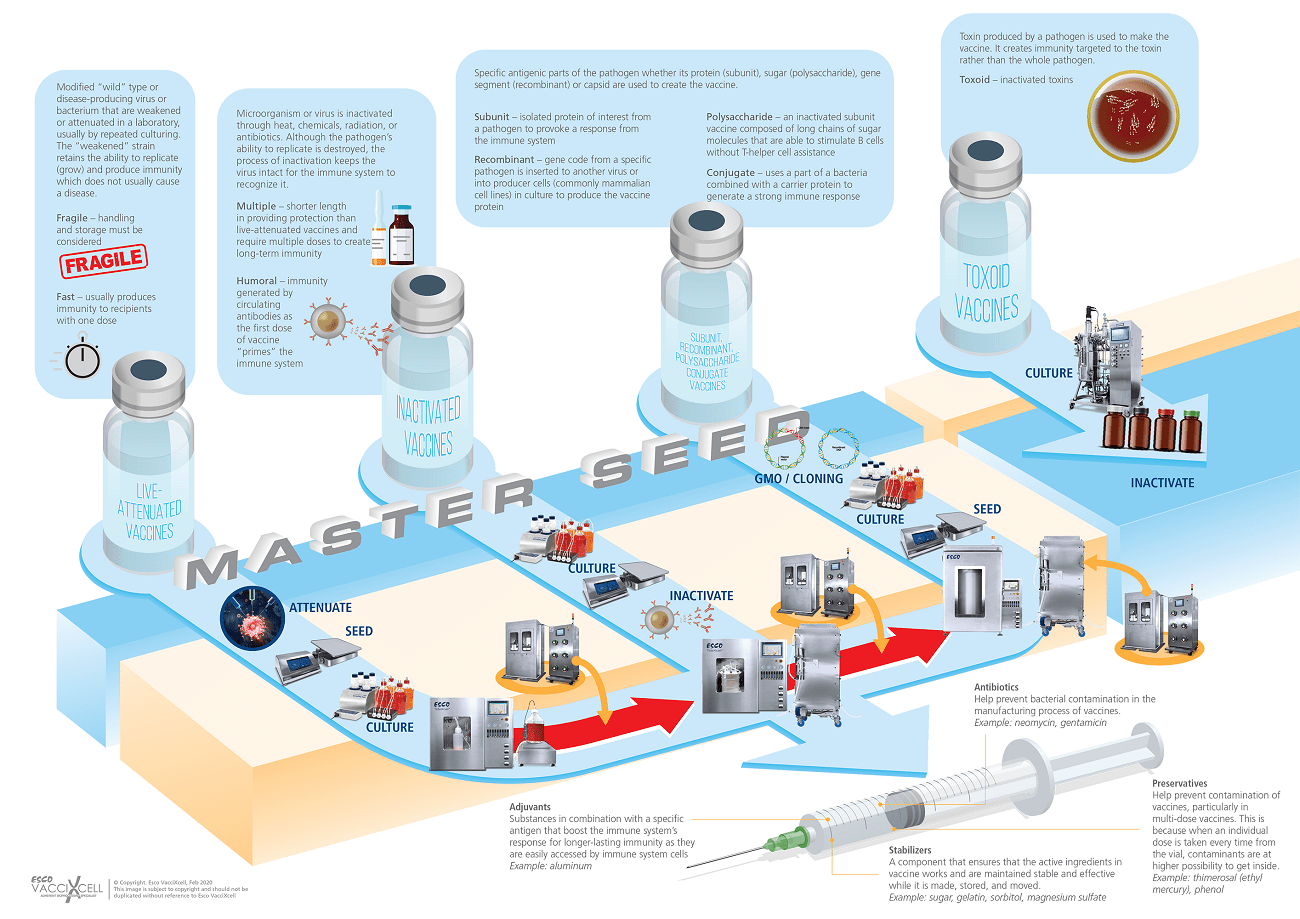
Inactivated and live-attenuated vaccine efforts have not yet been evaluated. Incomplete inactivation or handling live SARS-CoV-2 presents public health threat as it requires the propagation of high titers of infectious virus, which in the case of SARS-CoV-2, requires a biosafety level 3-enhanced precautions. While the race is on, certain bioprocessing equipment can fasten up the vaccine production. Bioreactors, for example, enables virus amplification as well as to yield higher secreted products needed to create the vaccine (Fig. 3). From small scale, to manufacturing scale, the linearity of the process reduces time constraints when scaling-up, using the same parameters with a higher product yield.
References:
Czub M., et al. (2005) Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine. 23: 2273-2279.
Hansson, M., et al. (2000) Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem 95-107.
Lanying Du, et al. (2009) The spike protein of SARS-CoV - a target for vaccine and therapeutic development. Nat Rev Microbiol. 7(3): 226-236.
Laura Gillim-Ross, et al. (2006) Emerging Respiratory Viruses: Challenges and Vaccine Strategies. Clin Microbiol Rev. 19(4): 614-636.
Nascimento, IP. (2012) Recombinant vaccines and the development of new vaccine strategies. Braz J Med Biol Res, Vol 45(12) 1102-1111.
Shibo Jiang, et al. (2005) SARS Vaccine Development. Emerg Infect Dis. 11(7): 1016-1020. Vaccines 2014, 2, 624-641; doi:10.3390/vaccines2030624
Ura, T. et. al. (2014) Developments in viral vector-based vaccines.
Van der Loo, J. et. al. (2015) Progress and challenges in viral vector manufacturing. Human Molecular Genetics Vol. 25. DOI: 10.1093/hmg/ddv451
Warnock, J. et al. (2006) Cell culture processes for the production of viral vectors for gene therapy purposes. Cytotechnology 50:141-162
Weingartl H., et al. (2004) Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 78: 12672-12676.
Sign up to our newsletter and receive the latest news and updates about our products!
