The global impact of rotavirus infection in infants and young children affects child morbidity and mortality worldwide at a high rate. Although first identified in animals in the 1960s, rotavirus (RV) was later discovered through the examination of a part of the small intestine (duodenum) of children who have severe diarrhea.
Rotavirus is a member of the family Reoviridae which causes severe diarrhea or rotaviral gastroenteritis. The virus is usually localized to the small intestine and infects the mature villus epithelial cells.
It can spread through hands, water, food, or object contamination; and its infection sequelae involve dehydration, electrolyte disturbances, and even death.
Most rotavirus-related deaths, surmounting to more than 80% of the infected population are found in developing countries such as Southern Asia and sub-Saharan Africa. Most cases occur before or by the age of five.
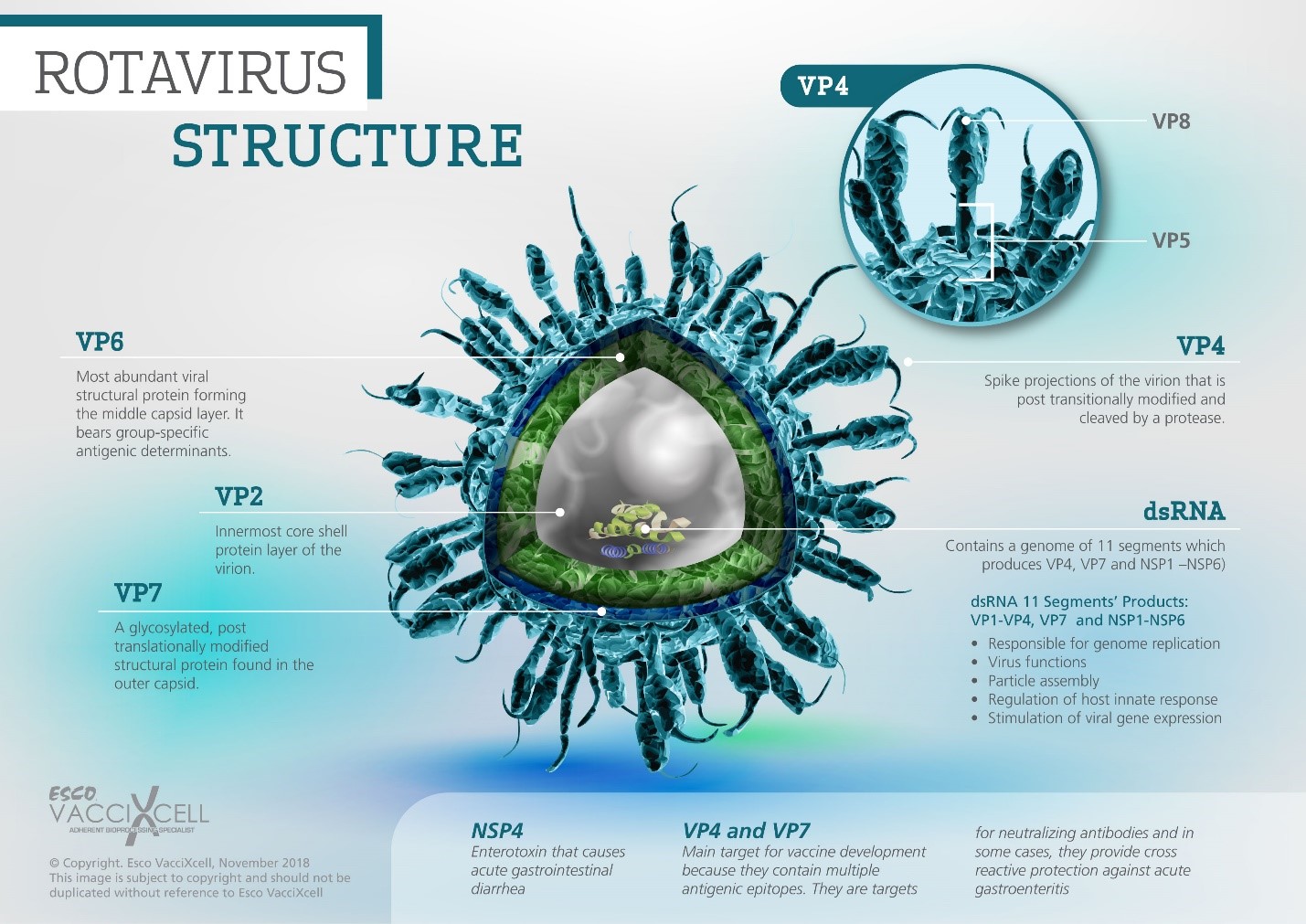
Figure 1. A part-by-part description of the tri-layered rotavirus structure
Rotavirus structure and mechanism
RV is a non-enveloped virus with icosahedral structure of 70 nm in size. Its name came from its “wheel-like” appearance wherein it contains 11 segments of double-stranded RNA (Fig. 1). These segments are responsible for the six structural proteins (VP1-VP4, VP6, VP7 structural and NSP1-NSP6 non-structural proteins) that make up the enclosed triple-layered capsid structure. The non-structural proteins are responsible for supporting various viral functions such as replication, regulation of host innate responses, and stimulation of viral gene expression. RVs replicate in mature enterocytes (intestinal absorptive cells) at the duodenum’s villus tips.
Serogroups A to G were described in which groups A, B, and C are human pathogens. From a public health standpoint, Group A rotaviruses are mostly important.
Acute dehydrating diarrhea in infants and children under the age of 5 are caused primarily by Group A RVs. Although significant number of children showed asymptomatic infections, the virus was shed in their stool and served as another possible source for the transmission of the virus. This contributed to the widespread retroviral infections even in older children and adults. Aside from humans, RVs are also responsible for gastroenteritis in common farm animals like cows, pig, or sheep. This also includes exotic animals, nonhuman primates, rodents, birds, and even house-hold pets.
The severity of the rotaviral disease differs from one child to the other; however, both industrialized and developing countries are similar in terms of rotavirus illness. This indicates that it is unlikely to prevent a disease with just clean water supplies or good hygiene as this have little effect on the transmission of the disease.
These reported high burden of rotavirus disease calls for urgently needed vaccines.
References:
About Retroviruses (RVs)
Retroviruses is a virus that mainly causes acute gastroenteritis and diarrhea, mostly in babies and young children. The virus sequelae include dehydration, vomiting, and fever. Source: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/rotavirus.html
About Tide Motion Bioreactors
Tide Motion pertains to the gentle oscillation of culture medium into and out of the matrix vessel that intermittently exposes the cells to aeration and nutrition. The upward oscillation exposes the cells to nutrition, while the downward oscillation exposes the cells to aeration. At the same time, this process washes away products and wastes. This oscillation produces no air bubbles and low shear stress. View a range of products at http://www.vaccixcell.com/tide-technology/
About Esco VacciXcell
Esco VacciXcell is the bioprocessing division of Esco Group of Companies that specializes in the marketing and manufacturing of bioprocessing equipment for cell culture.
Esco VacciXcell provides turnkey manufacturing solutions using its proprietary Tide Motion™ technology to help developing nations to be self-sufficient in the manufacturing, storing, distribution, and administration of vaccines and other biologics, thus providing a complete solution from Discovery to Delivery. For more information on VacciXcell, please visit www.vaccixcell.com
Sign up to our newsletter and receive the latest news and updates about our products!
