Global influenza epidemics emerge seasonally, and health authorities recommend that yearly vaccination is the most effective method for protection. Several vaccines such as inactivated, live-attenuated, quadrivalent, and pandemic influenza vaccines are available to target specific influenza strains. While this may be true, production of such vaccines has a limited timeframe depicting a crucial step for its release in the market. Vaccines can be produced faster through technologies available today however, period time for strain selection remains similar for all production platforms.
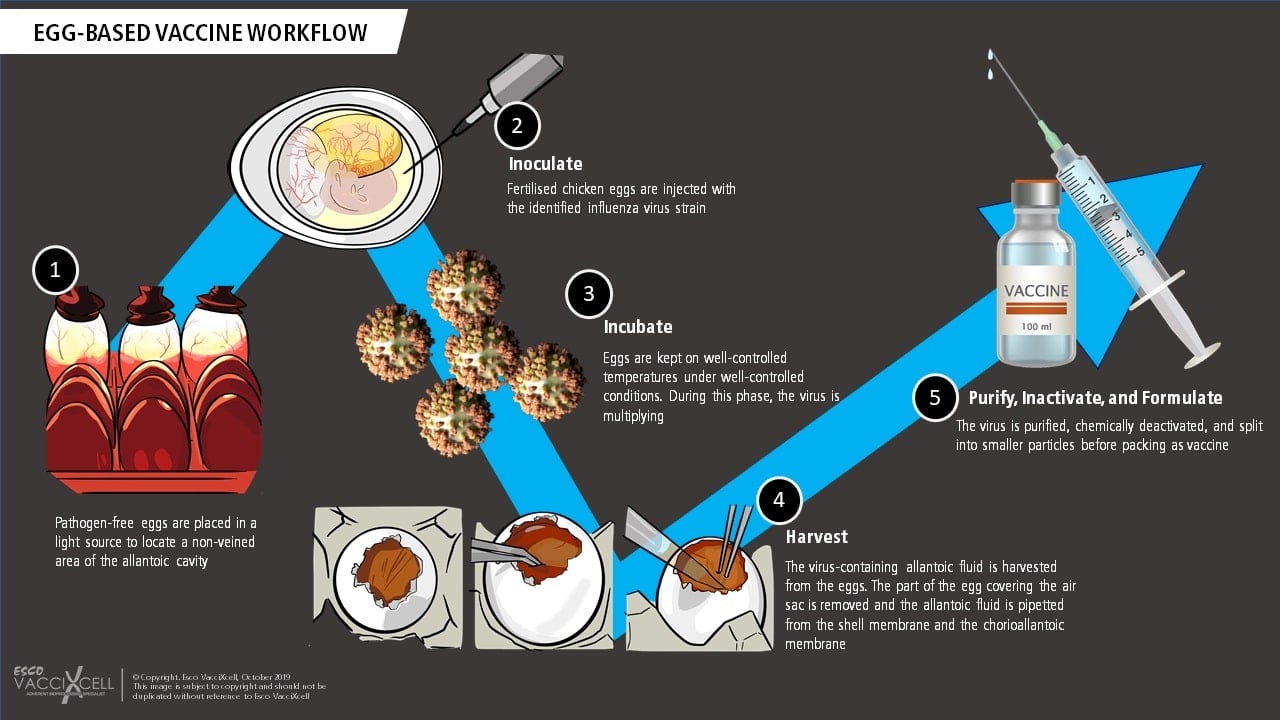
Egg-based vaccines are frequently used to prevent influenza. This involves using hundreds of thousands of embryonated chicken eggs to manufacture vaccines; beginning with the identification of the predicted virus strain. Quadrivalent vaccines, for example, are composed of IAV: H1N1 and H3N2 viruses, and IBV: Yamagata and Victoria viruses. A strain will be selected, and genetic segments will be reassorted into an egg-adapted virus. Once these viruses are generated, they will be injected into the eggs and screened to isolate "candidate vaccine viruses" or CVVs that are able to yield high viral titers and remain antigenic-like the circulating strains. The CVVs will then be mass-produced by vaccine manufacturers using eggs, allowing them to replicate inside. As a final step in vaccine production, the allantoic fluid of the egg will be purified.
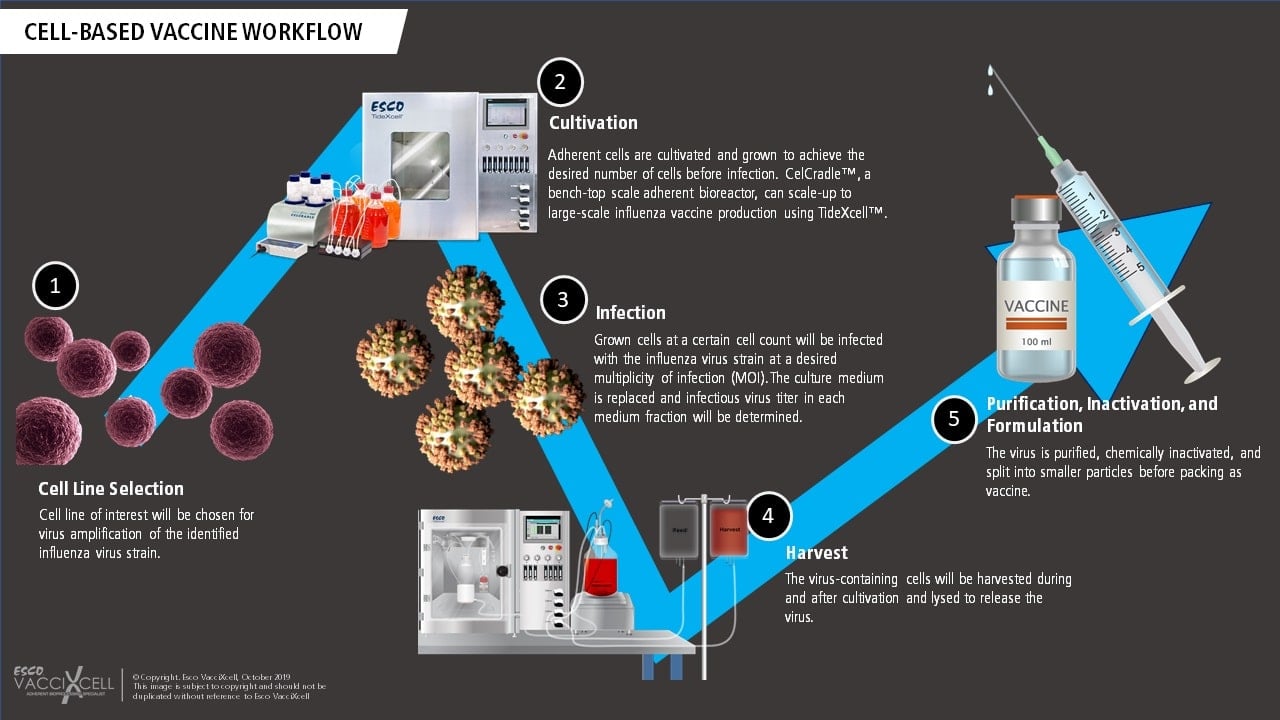
Cell-based vaccines such as the use of VERO and MDCK cell lines for viral production involve a controlled and adherent scale-up, closed manufacturing process. Influenza viruses, for example, are grown in cultured mammalian cells using two-dimensional (T-flasks, cell stacks) or in three-dimensional (bioreactors with microcarriers or macrocarriers) systems. This process is deemed flexible and provides faster timeline due to adherent scale-up using bioreactors. When producing live-attenuated cell-based vaccines, the adherent cell line of interest is chosen and screened for its virus amplification capability. The chosen adherent cells are then cultured in an in vivo-like environment before reaching confluency and infected with the virus. Tide Motion bioreactors are usually used for this application to produce high viral titer yield. The cultivation process continues for a few more days, afterwards, the virus-containing cells/fluid are harvested, inactivated using a highly stringent procedure (sometimes even split into smaller particles to further prevent replication), and purified to become a vaccine.
Both egg-based and cell-based platforms are used to create a vaccine against influenza virus. Still, the two have comparable differences in terms of how it is produced, how long it is made, and how flexible the process is.
Egg-Based VS Cell-Based |
||
|
Egg-Based |
Cell-Based |
|
|
Virus amplification |
Eggs |
Mammalian Cell Lines |
|
Manufacturing technique |
Hundreds of thousands of eggs |
2D systems to Bioreactors |
|
Production timeline |
6 months |
Within weeks |
|
Immunogenicity |
Antigenically mismatched |
Antigenically matched |
|
Vaccine scalability |
Scale-out |
Scale-up |
|
Containment capability |
BSL 2 |
BSL 3 to cGMP |
|
Allergy risk |
Egg allergies |
No risk |
Virus amplification through egg-based platform requires hundreds of thousands of eggs in order to produce lots of vaccine doses. Additionally, the timeline for this process will be made slower in the event of influenza pandemic caused by an avian virus, which is highly pathogenic to chickens.
Cell-based platform on the other hand, requires a stable cell line capable of propagating the virus and can scale up to manufacturing scale instead of replicating the amount of systems (scale-out) needed for vaccine production. Cell-based culture is more suitable for the growth of influenza strains rather than egg-based, as studies have shown that mammalian-derived is a representative of the natural virus. Adherent mammalian cell lines such as MDCK induce higher hemagglutination inhibition (HI) and neutralizing antibody (NAbs) titers than egg-based vaccine.
In the case of H5N1, WHO requires BSL 3 to produce a vaccine. The highest biosafety level available in egg-based vaccine manufacturing is BSL 2. The generation and safety testing of the procedure may take weeks and will result in the delay in the delivery of the vaccine. Cell-based solves this issue as VERO cell cultures allow high-titer growth of the said strain under BSL 3 conditions.
Cell-based vaccines use VERO and MDCK adherent cells lines which can both be cultured in cGMP Tide Motion bioreactors. The bioreactor utilizes the Tide motion principle that provides gentle oscillation of the medium, with an extremely low shear stress, foaming, and bubble-free environment for the cells. Both VERO and MDCK cells have been optimized in a culture system using CelCradle™ bioreactor that houses BioNOC™ II macrocarriers for cell growth which mimics 3D in vivo environment.
The expansion of adherent cells such as VERO and MDCK in the CelCradle™ can be achieved through linear adherent scale-up using the TideXcell™ bioreactor. This demonstrates that innovative technologies being developed today can improve the efficiency of historically established egg-based systems.
References:
Bruxvoort, K. J., Luo, Y., Ackerson, B., Tanenbaum, H. C., Sy, L. S., Gandhi, A., & Tseng, H. F. (2019). Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017-2018. Vaccine. doi:10.1016/j.vaccine.2019.08.024
Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD) (24 September 2018). How influenza (flu) vaccines are made. Retrieved from https://www.cdc.gov/flu/prevent/how-fluvaccine-made.htm
Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD) (11 October 2019). Cell-based flu vaccines. Retrieved from https://www.cdc.gov/flu/prevent/cell-based.htm
Harding, A. T., & Heaton, N. S. (2018). Efforts to Improve the Seasonal Influenza Vaccine. Vaccines, 6(2), 19. doi:10.3390/vaccines6020019
Hector S Izurieta, Yoganand Chillarige, Jeffrey Kelman, Yuqin Wei, Yun Lu, Wenjie Xu, Michael Lu, Douglas Pratt, Steve Chu, Michael Wernecke, Thomas MaCurdy, Richard Forshee, Relative Effectiveness of Cell-Cultured and Egg-Based Influenza Vaccines Among Elderly Persons in the United States, 2017-2018, The Journal of Infectious Diseases, Volume 220, Issue 8, 15 October 2019, Pages 1255-1264, https://doi.org/10.1093/infdis/jiy716
Sign up to our newsletter and receive the latest news and updates about our products!
