Hayflick and Jacobs have established and developed human diploid cell strains (HDCSs) for Wistar Institute (WI)-38 and Medical Research Council (MRC)-5, respectively, over 50 years ago for vaccine production. Since then, these two cell strains have been accepted as standards for HDCSs and licensed worldwide for the production of vaccines including: inactivated and oral polio vaccine, rabies, rubella, measles, chicken pox, mumps, and hepatitis A. Applications for HDCSs extend to antitumor viruses, as oncolytic viruses, including adenoviruses which have been seen to inhibit the growth of breast cancer, are reported to also be generated from the cell strains.
The valuable characteristics of HDCSs for vaccine production include: susceptibility to a wide range of viruses, high number of cells, long storage potential, low risk of adventitious agents, low cost of cell procurement from cell banks, and good safety profile. The cell strains have also been well-characterized to be non-tumorigenic and thus, considered safe for production of vaccines. World Health Organization recommends HDCSs as the safest cell substrates for vaccine production.
The main disadvantage of HDCSs, however, is that the cells have limited life span for in vitro propagation. Since the original cell strains were obtained from human fetal abortions, ethical reviews and strict requirements thwarted the development of back-up strains. Despite this, vaccine manufacturers still prefer HDCSs as substrates for vaccine manufacturing.
Scientists from Wuhan University and Yunnan Walvax Biotechnology in China established a new human diploid cell line to solve the challenges posed by the use of HDCSs. Before the study, most of the HDSCs originated from internationally. Also, the imported cell strains which were seen to be later than 20th passage reduced its ability to grow. Hence, the team established a new cell strain, walvax-2, capable of cultivating viruses on par with MRC-5 for rabies, hepatitis A, and varicella. Tumorigenicity test also showed little risk for potential carcinogenesis upon injection on humans.
Despite the development of back-up strains, the limited in vitro life span of HDCSs will remain a challenge for vaccine manufacturers. Even the source of these new back-ups remains controversial and would still require strict reviews in the coming years. Thus, it is beneficial to maximize the potential of the stored cell banks of passaged WI-38 and MRC-5, as well as other candidate back-ups.
WI-38 and MRC-5 are adherent cell lines which can be cultured in Tide Motion bioreactors. The bioreactor utilize the Tide motion principle that provides gentle oscillation of the medium, with an extremely low shear stress, foaming, and bubble-free environment for the cells. MRC-5 cells have been optimized in a culture system using CelCradle™ bioreactor. The cells grow on BioNOC™ II macrocarrier that mimics 3D in vivo environment. Batch mode culture (7-8 days) using Tide Motion bioreactor and macrocarrier generated 19-fold increase from seeding density of 15,000 cells/carrier.
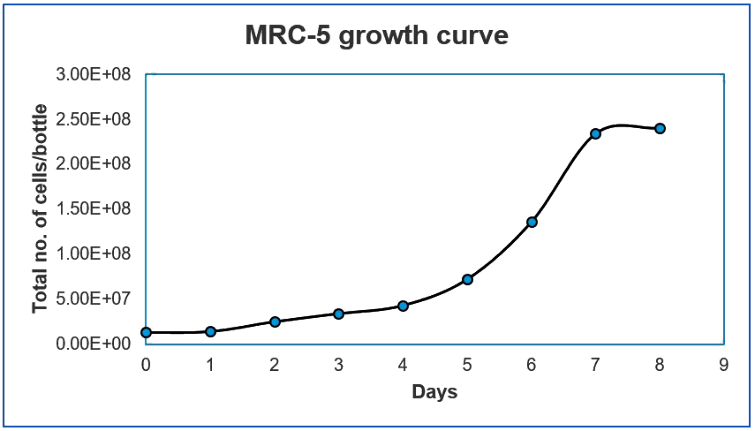
Figure 1. Growth curve of MRC-5 cells in CelCradle™ bioreactor
The expansion of HDCSs in the CelCradle™ for scale-up production can be achieved through linear scale up through the TideXcell™ bioreactor. This demonstrates that innovative technologies being developed today can improve the efficiency of historically-established systems.
References:
Leiva, Rene. (2006). A brief history of human diploid cell strains. The national Catholic bioethics quarterly. 6. 443-51. 10.5840/ncbq20066328.
Ma, B., He, L. F., Zhang, Y. L., Chen, M., Wang, L. L., Yang, H. W., ... Zheng, C. Y. (2015). Characteristics and viral propagation properties of a new human diploid cell line, Walvax-2, and its suitability as a candidate cell substrate for vaccine production. Human vaccines & immunotherapeutics, 11(4), 998-1009. doi:10.1080/21645515.2015.1009811
Zhu, W., Wei, L., Zhang, H., Chen, J., & Qin, X. (2012). Oncolytic adenovirus armed with IL-24 inhibits the growth of breast cancer in vitro and in vivo. Journal of experimental & clinical cancer research: CR, 31(1), 51. doi:10.1186/1756-9966-31-51
Sign up to our newsletter and receive the latest news and updates about our products!
