Biomanufacturing companies have long sought better approaches with regards to bioprocessing. Due to rapidly fluctuating market demands for biologics, there is a growing interest in the benefits of continuous bioprocessing, mainly for cell culture. This process had increasingly driven biomanufacturing companies to develop innovative solutions for highly flexible and cost-effective production.
THE DIFFERENCE:
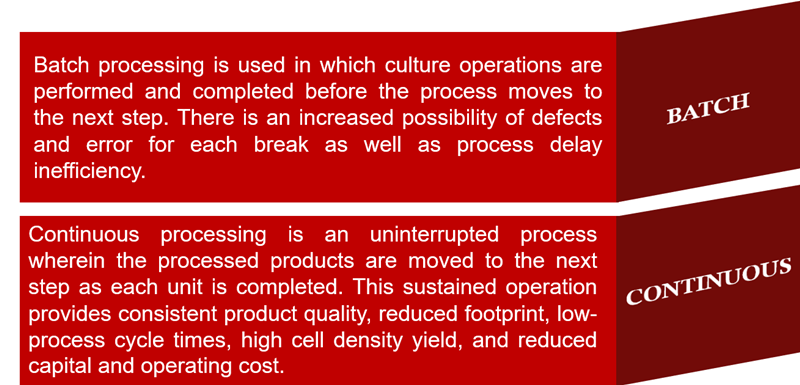
FDA urges biomanufacturing companies to transit from batch to continuous processing as it allows them to respond to the market and contributes to the prevention of drug shortages. Although the adoption of continuous processing is slow, the benefits include reduced cost, increased productivity, improved quality, and increased flexibility. Esco VacciXcell’s TideCell® unit operations are continuous and integrated transforming bioprocessing in a faster and more reliable process mode.


TideCell requires smaller footprint and will require less manpower

PLC-based monitoring and control system. As a default, it can be connected and controlled using WonderWareSCADA

Proven to be linearly scalable and can either run in normal or hypoxic conditions. TideCell also has 90% recovery rate using the proprietary TideCell Cell Harvesting System
Operates at high cell culture viability, which results to higher cell density yield, lower impurity levels in harvest, and simplified product purification schemes

100% media exchange system and zero shear stress conditions making it ideal for culturing difficult viruses
It has been a long time and drugs are still made on a traditional basis. By drawing upon the experience and urge of the FDA to transit from batch to continuous, enhancing the quality of how drugs are made will provide a framework and a guiding principle for a better and more reliable manufacturing.
Learn more about TideCell here: http://www.vaccixcell.com/
Sign up to our newsletter and receive the latest news and updates about our products!
