One of the significant trends this 2019 is the expanding research in immunotherapy. As one of the hot topics, the development and manufacturing of chimeric antigen receptor (CAR) T-cell therapy pipelines will continue to progress towards commercialization.
CAR-T therapy is an autologous oncolytic treatment that is created by extracting the T cells from the patient's blood and reintroduced to the patient after undergoing a modification process in the laboratory. These drugs cannot be mass produced because of its complex and long process.
Because of this, manufacturers are aiming for the off-the-shelf CAR-T or also known as allogeneic therapy. These allogeneic CAR-T therapy uses cells from healthy donor to prompt the same immune response as the first-generation CAR-T, thus, a combination of gene editing tool like CRISPR Cas9 is needed to achieve efficient and specific multiplexed editing.
Production of Allogeneic CAR-T Therapy
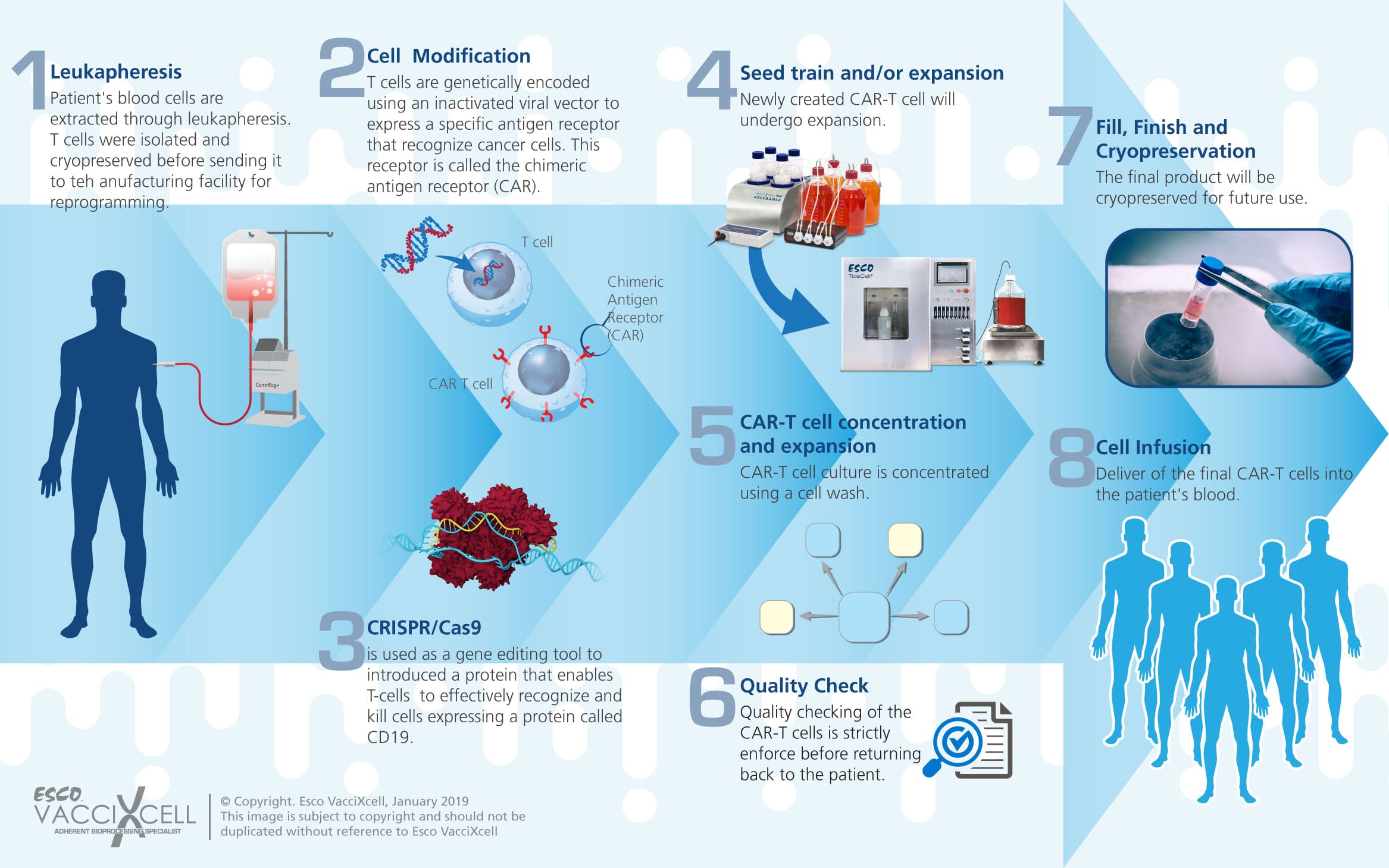
Allogeneic CAR-T manufacturing process is less complex and more cost effective for mass production.
Esco VacciXcell is continuously providing the best bioprocessing tools for immunotherapy - from the process of mass production to harvesting of cells with the use of TideCell® and TideCell® Cell Harvesting System, respectively.
The expansion of reprogrammed CAR-T cell is essential to meet the growing demands in the health industry, especially in treating cancer and other types of diseases. There are several steps in the production of CAR-T cells, and strict quality control testing is required in the production of CAR-T cell therapy. It is important to establish quality control testing for safety, sterility, potency, identity, and titer to ensure that each batch of vector meets defined standards before it is used to transduce T cells.
Reference/s:
Investor's Business Daily (21, November 2018). Here's How Biotechs Plan to Attack Cancer, Other Disease in 2019. Last Accessed 3 January 2019 from https://www.investors.com/news/technology/biotech-companies-2019-crispr-g ene-editing-immunotherapy/
Genetic Engineer & Bioteacnology News (30, December 2018). 5 Biopharma Trends to Watch in 2019. LAst Accessed 3 January 2019 from https://www.genengnews.com/lists/5-biopharma-trends-to-watch-in-2019/
About CAR-T Therapy
CAR-T therapy a type of treatment in which a patient's T cells (a type of immune system cell) are changed in the laboratory so they will attack cancer cellsCAR T-cell therapy is being studied in the treatment of some types of cancer. Also called chimeric antigen receptor T-cell therapy.
Reference: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/car-t-cell-therapy
About Tide Motion Bioreactors
Tide Motion pertains to the gentle oscillation of culture medium into and out of the matrix vessel that intermittently exposes the cells to aeration and nutrition. The upward oscillation exposes the cells to nutrition, while the downward oscillation exposes the cells to aeration. At the same time, this process washes away products and wastes. This oscillation produces no air bubbles and low shear stress. View a range of products at http://www.vaccixcell.com/tide-technology/
About Esco VacciXcell
Esco VacciXcell is the bioprocessing division of Esco Group of Companies that specializes in the marketing and manufacturing of bioprocessing equipment for cell culture.
Esco VacciXcell provides turnkey manufacturing solutions using its proprietary Tide Motion™ technology to help developing nations to be self-sufficient in the manufacturing, storing, distribution, and administration of vaccines and other biologics, thus providing a complete solution from Discovery to Delivery. For more information on VacciXcell, please visit www.vaccixcell.com
Sign up to our newsletter and receive the latest news and updates about our products!
