Upstream processing with adherent cells starts with culturing a chosen adherent cell line, a virus type, and targeting what the product will be. For processes involved using suspension cell lines,different cell culture techniques have been optimized for different applications.This varies from vaccine manufacturing to biotherapeutics which require the specific design of upstream and downstream processing. An efficient, reliant, and scalable technology is needed to produce these products.
Critical upstream processing operations such as seeding and culturing can seamlessly be done if the said technology can scale-up from research and development, into clinical trials, and pilot or production cGMP manufacturing. In addressing considerations to come up with the right platform technology for your cell culture, whether using adherent cells or suspension cells, it comes down to cost, quality and quantity, and scalability.
Most research today include virological, cell growth, replication, proliferation, cell life cycle, virus-host interaction, and disease modelling to develop drugs. Most of what is known today came from accounts of different studies of adherent cultures in 2D systems, in which cells are grown on flat surfaces such as flasks or petri dish, or on animal model systems. However, animal models involve a lot of cost-intensive factors and have been a question in the industry due to their ethical use. In terms of chosen cell line, adherent cell lines offer better models in cytology as compared to suspended-adapted mammalian cell lines.
| Adherent Culture | Suspension Culture | |
|---|---|---|
| Cell type |
Applicable for most cell types, including primary culture
(VERO, Stem Cells, and more) |
Applicable for adapted cell lines for suspension culture and others
(CHO, insect cell lines, and more) |
| Passage |
Requires dissociation enzyme/process (chemical or mechanical) |
Requires dilution or splitting *others do not require chemical or mechanical dissociation |
| Cell Growth | Limited to surface area | Limited to cell concentration |
| Culture vessel | Requires tissue-culture treated vessel for surface attachment | Maintained in non-treated culture vessels but requires agitation |
| Application | Usually used for cytology, drug screening, disease modeling, secreted and non-secreted products for various applications (R&D, Vaccines, Cell Therapy etc.) and more | Bulk production, batch harvesting, and more |
Parameters and protocols for non adherent culture using suspension cells have been long established making culture less complicated than culturing adherent cells. Since cells are free-floating (suspended) in the growth culture medium, no enzymatic dissociation reagents are required to detach cells from the culture vessel’s surface. The whole process is fast; however, culture medium replacement cannot be carried out due to its nature of culture. The optimum growth requirements are maintained through feeding suspension cells with fresh medium every 2-3 days until they reach confluency.
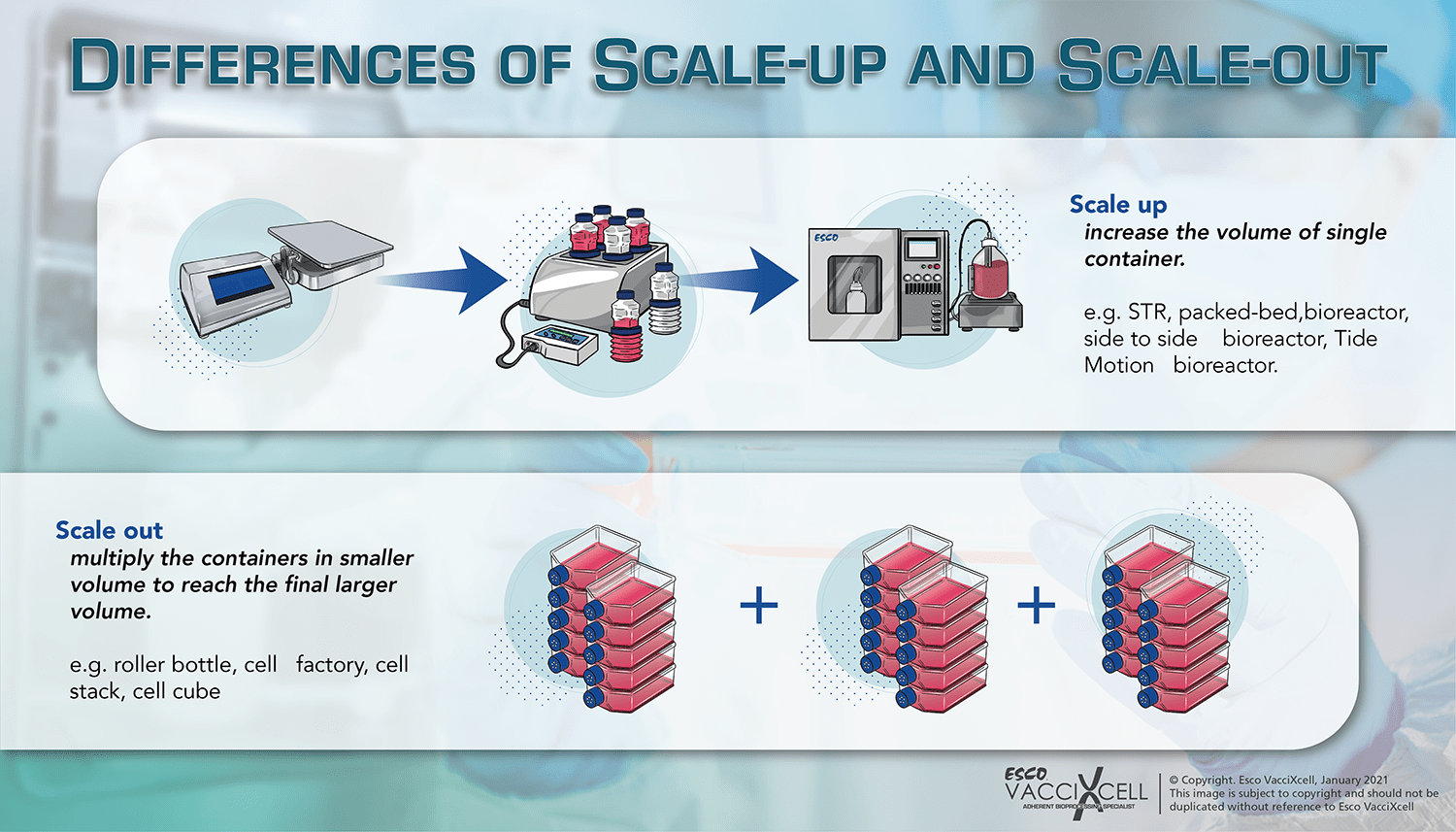
For adherent cells production, while cells grown in two-dimensional adherent cultures grow in a monolayer culture flask or petri dish and attaches to the surface of the system, three-dimensional cultures imitate the architecture of the parental tissue more accurately compared to 2D culture systems. These systems also have the capability to scale up rather than scale out.
Typically, adherent cells are grown as monolayers in static cell culture systems such as Petri dishes and T -flasks. T-flasks vary in size and can provide a surface area from 25 cm2 to 225 cm2 for cell culture. They are generally used for subculture, generation of seed material for small-scale and large-scale productions. In the case that a larger surface area is needed, more t-flasks are usually used. Scaling out using T-flasks however, is labor intensive and takes up more incubator space.
Multi-tray systems, also known as cell factories or cell stacks, have been developed for cell culture systems that require surface area up to 25,400 cm2. They provide cells with a large multi-level surface area for adherence and growth with trays stacked one above the other.
While a cell factory can overcome the limitations of T-flasks in terms of scaling out, it is essentially still a large T-flask with multiple layers. Hence, it remains a traditional static system with no agitation, aeration, or movement of media. There are also concerns with regards to possible differences in the gaseous exchange between the middle unit and top or bottom units. Significant differences could lead to variability in yield and quality of cells.
Roller bottles have been widely used for applications of biotechnology, particularly in the development of vaccines. Unlike static systems, roller bottles allow agitation of the media (through roller automation) and prevent formation of gradient that can adversely affect the cells. They can also provide a larger surface area than the standard T-flasks and are much easier to use than cell factories but require an additional device that would facilitate rotation of roller bottles.
Automation is recommended when many roller bottles must be utilized. Though automated roller bottle systems can potentially provide surface area of more than 350,000 cm2, the process tends to be labor intensive as number of roller bottles needed increases.
|
Existing 2D Culture Systems |
BioNOC™ II 3D Culture |
|
Single layer cell shape |
Multiple layer cell shape |
|
Morphology is sheet-like flat and stretched in monolayer |
Morphology from aggregate or spheroid structures |
|
Cell to cell contact is limited |
Physiologic cell to cell contact dominates |
|
Cells contact extracellular matrix only on the surface |
Cells interact with extracellular matrix (ECM) |
|
Culture unable to establish a microenvironment |
Culture mimics an in vivo-like environment |
|
Displays differential gene/protein expression as compared with in vivo models |
Gene and protein expression levels in vivo present |
|
Limited surface area available for growth at a given volume |
Larger surface area capable of culturing up to 109 cells per gram |
|
Only offers incremental increase in the SA/V |
Dramatic increase in SA/V |
Microcarriers are particles to which adherent cells can attach and grow suspended in a stirred tank bioreactor. Compared to the mentioned static systems, microcarriers can significantly provide surface area for large-scale production. However, some issues such as shear stress and uneven oxygen distribution, that often arise when using microcarriers, can significantly affect the quality and yield of cells. Some microcarriers uses glass, fibers or more as its main material of construction. The difference in topography should be carefully examined before choosing the microcarrier suitable for the production.
The wave bioreactor makes use of rocking movement to agitate the cells. It can provide good nutrient distribution, efficient oxygen transfer, and extremely low shear stress. The main disadvantage though of the wave bioreactor is its limited scalability. Rather than scaling up to the next volume, the system has to be scaled out to get the target product in the end.
Macroporous carriers are fibrous scaffolds that supports adherent cell growth giving off an in vivo-like environment. They provide higher surface area to volume ratio as compared to microcarriers. For mesenchymal stem cell culture, macroporous carriers provide better cell anchorage and have specific indentations for higher oxygen intake. The sizes of the carrier’s pores enable increased protection from shear stress done during agitation, as well as when introducing gases through spargers for controlling several parameters.
Packed bed bioreactors offer large surface area and can produce high cell densities (0.5-2 x 108 c/ml carrier). The main drawbacks of the system though, include limited scalability due to concentration of gradients over the fixed bed and generation of a nutrient/oxygen/CO2 gradient over the height of the fixed bed resulting to a non-homogeneous environment. The agitation principles in a fixed-bed or packed-bed varies from one type of bioreactor to the other.
|
Microbeads |
Macroporous Carriers |
|
|
Culture Process |
Relatively Simple |
Involves additional steps esp. harvesting (for some types) |
|
Cost |
Inexpensive; but others require specialized equipment |
More expensive |
|
Agitation Approach |
Stirred |
Upward Downward (Tide Motion), Side to Side, Fountain |
|
Shear Stress |
Higher as culture system scales up |
Extremely low sheer stress due to gentle mixing |
|
Surface Treatment |
Surface-coating required to avoid cell attachment on side surfaces |
No culture vessel coating required |
|
Scalability |
Large-scale production achievable |
Large-scale production achievable
|
|
Cell Growth |
Cell Size and Shape Variability |
3D growth in in vivo |
|
Cell Sampling |
Problematic if microcarrier aggregates form |
Visual sampling and staining for cell observation possible
|
|
Cell Imaging |
Imaging depends on scaffold size |
Possible through macrocarrier sampling |
|
Waste |
Aggregates form |
Low lint waste |
The important process in considering carriers for your next adherent cell culture involves selecting one that can create the highest product and cell yield in the most cost-effective way without compromising cell quality. Downstream processing steps should also be considered as well as the technology’s limitations when expanding and processing cells. Using these considerations, an optimal process workflow can be devised.
Bioprocessing involves a lot of applications from vaccine manufacturing to stem cell therapy. The central concept through which these products may be made is through the use of current technologies, not only to speed up the development process but also to maximize yield at the end of the production. The roots of current-day culture of different adherent cells are based on research and development scale that would later on expand for clinical trials and manufacturing. In order to cater such large-scale expansion, choosing the right platform is important for future success.
Tide Motion bioreactors involve a proprietary method that cultures cells at a gentle upward and downward motion for alternate nutrition and aeration exposure. It uses the heart of the Tide Motion bioreactor, BioNOC™ II as a material where adherent cells can grow and maximize its given growth surface area. Both the principle and the macroporous carrier work to fundamentally impact the targeted cell or product yield at large quantities at a lowest cost.
As different processes involve different workflows, Tide Motion enables the culture of adherent cells to produce much higher and needed cell numbers due to its linear scalability, automation, and increased process control.
Sign up to our newsletter and receive the latest news and updates about our products!
