Chimeric antigen receptor (CAR) – T cell therapy is the first FDA approved immunotherapy to cure cancer. T cells are the backbone of this therapy because they are the killer cells of our immune system that fight foreign substances such as bacteria, viruses, or tumor cells. CARs are engineered receptors that allow T cells to recognize and attach to a specific protein or antigen on tumors. Compared to bone marrow transplants, this T cell therapy bypasses the MHC-dependence of T cell receptor signaling requirements, thus, large percentage of potential cancer patients can be treated with this therapy.
Due to continued progress of this treatment last May 1, FDA approved another autologous CAR-T therapy called tisagenlecleucel. Tisagenlecleucel is used to treat refractory (does not respond to any forms of treatment) or relapse (recurrence of a past condition) diffuse large B-cell lymphoma (DLBCL) and B-cell acute lymphoblastic leukemia (ALL). According to the FDA, approximately 30% of patients are affected with DLBCL effects an estimated 27,000 newly diagnosed cases each year.
With the proven effects of this novel therapy, refractory and relapse DLBCL and ALL may now be treated.
Mechanism of Action: a transgene which expresses a chimeric antigen receptor that can reprogram the patient's T cell. Once it is reprogrammed, T cells would be able to identify and eliminate CD-19 expressing malignant and benign cells.
Production of CAR-T therapy.
The expansion of reprogrammed CAR-T cell is essential to meet the growing demands in the health industry, especially in treating cancer and other types of diseases. There are several steps in the production of CAR-T cells, and strict quality control testing is required in the production of CAR-T cell therapy.

Figure 1: Allogeneic CAR-Immune Cell Therapy Workflow Process
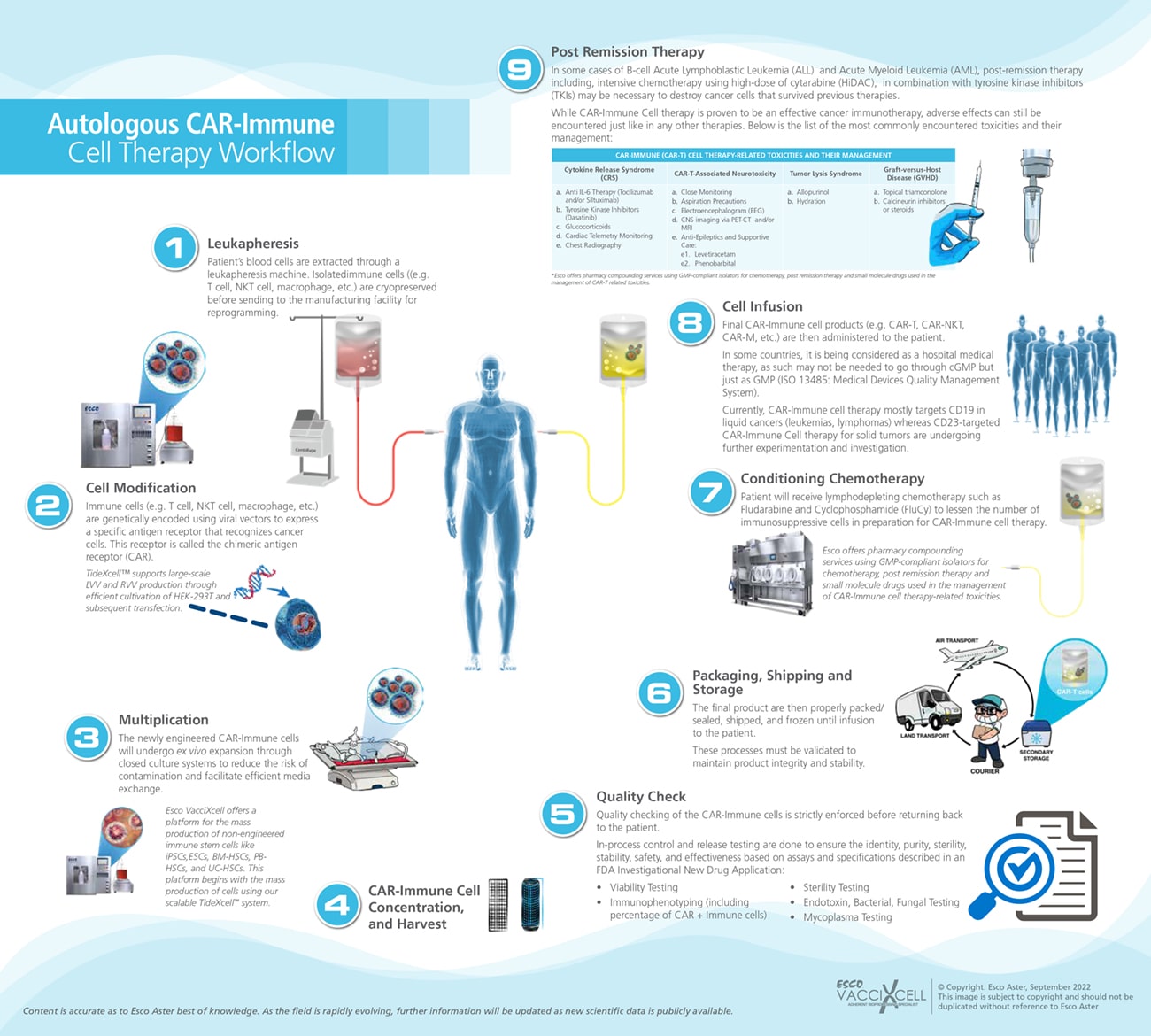
Figure 2: Autologous CAR-Immune Cell Therapy Workflow Process
the generation of sterile viral vectors is the most crucial part in producing CAR-T cell, because the final CAR-T cell product cannot be sterilized by filtration. With this, adherent HEK-293T cells are commonly used to produce large quantities of replication-defective viral vectors. A closed system cell processing and a good manufacturing practice (GMP) facility are required to maintain the sterility of the viral vectors.
To this end, bioreactor culture systems are designed to mimic the required environment for the proliferation of this viral vector. Using VacciXcell Tide Motion Technology, CelCradle™ bioreactor specializes in supporting high cell growth density for many anchorage-dependent lines including HEK-293T. With a high aeration and nutritional support, cells can produce infectious viral titer up to 109 IFU/ bottle to meet up with a demand needed in engineering CAR-T cells.
It is important to establish quality control testing for safety, sterility, potency, identity, and titer to ensure that each batch of vector meets defined standards before it is used to transduce T cells.
Reference:
EscoVacciXcell
21 Changi South Street 1
Singapore 486777
T: +65 6542 0833
E: [email protected]
About CAR-T Therapy
CAR-T therapy a type of treatment in which a patient's T cells (a type of immune system cell) are changed in the laboratory so they will attack cancer cellsCAR T-cell therapy is being studied in the treatment of some types of cancer. Also called chimeric antigen receptor T-cell therapy.
Reference: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/car-t-cell-therapy
About Tide Motion Bioreactors
Tide Motion pertains to the gentle oscillation of culture medium into and out of the matrix vessel that intermittently exposes the cells to aeration and nutrition. The upward oscillation exposes the cells to nutrition, while the downward oscillation exposes the cells to aeration. At the same time, this process washes away products and wastes. This oscillation produces no air bubbles and low shear stress. View a range of products at https://escovaccixcell.com/
About Esco VacciXcell
Esco VacciXcell is the bioprocessing division of Esco Group of Companies that specializes in the marketing and manufacturing of bioprocessing equipment for cell culture.
Esco VacciXcell provides turnkey manufacturing solutions using its proprietary Tide Motion™ technology to help developing nations to be self-sufficient in the manufacturing, storing, distribution, and administration of vaccines and other biologics, thus providing a complete solution from Discovery to Delivery. For more information on VacciXcell, please visit https://escovaccixcell.com/
Sign up to our newsletter and receive the latest news and updates about our products!
