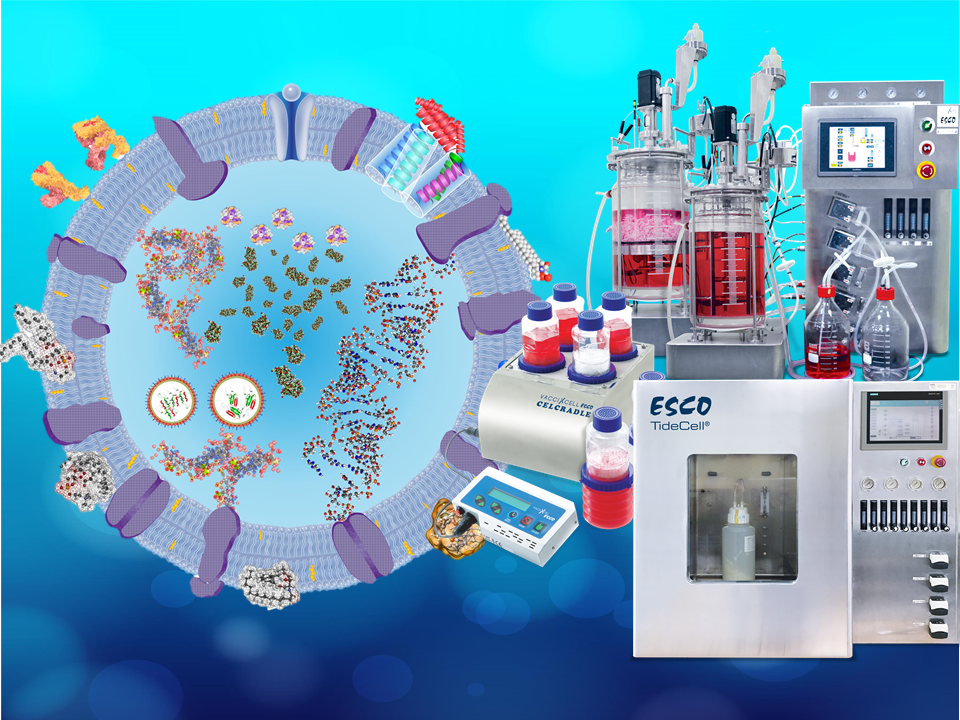
Exosomes provide promise in its potential for therapeutic application. The role of these extracellular vesicles (EVs) as a nano-vehicle for transporting molecular payloads, is designed to treat diseases. For example, catalase incorporated in exosomes, targets and delivers this cargo to the brain tissue, leading to a promising option for Parkinson's Disease (PD) therapy.
These nanovesicles have also been utilized in many studies and with proven therapeutic potential in the repair of cardiac tissue after heart attack. Exploration on exosomes' prognostic potential are underway, especially for predicting cell therapy outcomes.
Although a promising therapeutic platform, exosomes face challenges regarding their productivity. Exosome yield of 10-100 µg is needed to become a useful therapeutic drug; however, exosomes typically yield 1µg exosomal protein from 1mL of culture medium. The current practice for producing exosomes involve a culture of a cell line of interest where the exosomes will be derived from, collection or harvest of the conditioned medium, and purification.
The culture, harvest, and purification process have been reported in a handful of studies either been undertaken or are currently ongoing. Cells currently employed in manufacturing exosomes include the use of autologous, modified dendritic-derived exosomes, allogeneic mesenchymal stem cell (MSC)-derived exosomes, and autologous plasma-derived exosomes amongst others.
Exosomal Production
Exosomes are the secreted products of cells from where it is derived from. Given that cells produce and secrete these EVs naturally, the exosomal manufacturing platform should produce large quantities of these cells without altering its phenotype.
Research efforts have focused on scalable systems that have a large surface area and offer a greater process of control for culturing cells that will secrete the desired exosomes. Moreover, another main technical limitation of existing technologies that had been put in extremes is shear stress. On-going studies opt to resolve the scale-up transition problems of traditional systems (2D systems) to larger expansion systems, which alters the composition and function of the exosomes.
For example, MSC-derived exosomes moving from static, planar to 3D environment culture lead to shear stress. This alters the capability of MSCs to produce target therapeutic exosomes due to osteogenic differentiation. T cells, which were agitated at 180 rpm in bioreactors, caused impairment and exhaustion. T cells lose their reactivity and therefore loses its function to produce Interleukin 2 (IL-2). This IL-2 is a critical factor that performs elimination of cancer and virus-infected cells.
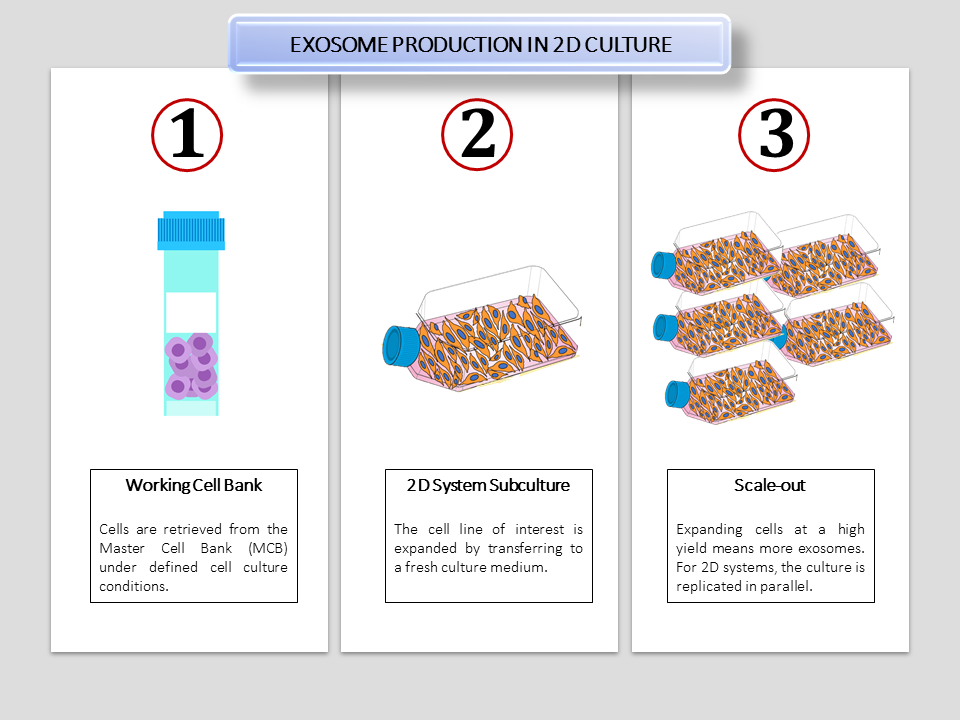
Figure 1. A schematic showing the expansion of exosomes derived from a cell line of interest in traditional 2D culture systems. This current technology shows limitations in scale-up, as it is manually operated and has a low process control. Operator errors may also cause contamination risks.
Current production platforms such as the use of T-flasks or multiple stacks for scale out, provided pitfalls due to its limited surface area. The use of conventional culture systems limits culture expansion at a higher density, as well as requiring large volumes of culture medium to process.
The robust expansion of the parent cell line requires a culture medium that reduces process-related contaminants and supports exosome release. In most cases, fetal bovine serum (FBS) contains significant amount of endogenous exosomes that can alter and contaminate the secreted exosome. This heavy reliance on animal serum for cell growth is completely unfavorable. A regulatory standpoint that exosomes make it into the final drug product as an injectable, concurs limitation of the process.
An option of using serum-free medium (SFM) had been studied to replace the use of FBS; however, not all platform cell line performs well. Some cell lines being cultured with SFM, have been observed to yield exosomes that contain reactive oxygen species and stress-related proteins.
The Tide Motion Platform
One significant advantage of generating exosomes as products, is that the exosome-rich conditioned medium may be harvested from its producer cell lines (most specifically stem cells). Stem cells are typically adherent in nature, eliminating the need to enzymatically detach the cells from the carriers.
With the Tide Motion platform, most of the limitations faced when manufacturing exosomes are solved. The Tide Motion technology provide extremely high surface-to-volume ratios for culturing cell lines at a high density. The heart of the Tide Motion bioreactors, BioNOC™ II, acts as the packed bed component of this system and has enough surface area to culture up to 10 billion cells per gram. This carrier is made of 100% PET which generates low lint waste at the end of the production, easing purification steps. BioNOC II is configured in 3D, which mimics the in vivo environment of the platform cell line. Cells are able to grow on the surface of the fibers, as well as the spaces in between them. As highly porous carriers, nutrients and oxygen can penetrate the bed, retaining cell lines to grow to post-confluence.
The proprietary Tide Motion relates to the cyclical high and low tide of the bodies of water on earth. Like the tides, Tide Motion is the gentle upward and downward movement of the culture medium in the bioreactor. With its gentle oscillation, shear stress during culture is eliminated. Given that cells secrete exosomes in the extracellular milieu, the Tide Motion bioreactors also offer perfusion-based cultures and 100% media exchange for adequate mass transfer. Production can be done continuously for over several months. These approaches retain the exosome product within the culture compartment, yielding to a more concentrated conditioned medium.
Notably, the Super Plus™ overcomes the serum limitation for exosome production. Super Plus is a cell culture supplement designed to reduce or eliminate the requirement for serum in the basal medium. This culture medium also minimizes the culture variance due to inconsistent quality between serum batches. No serum-endogenous exosome interface will be observed when culturing cells.
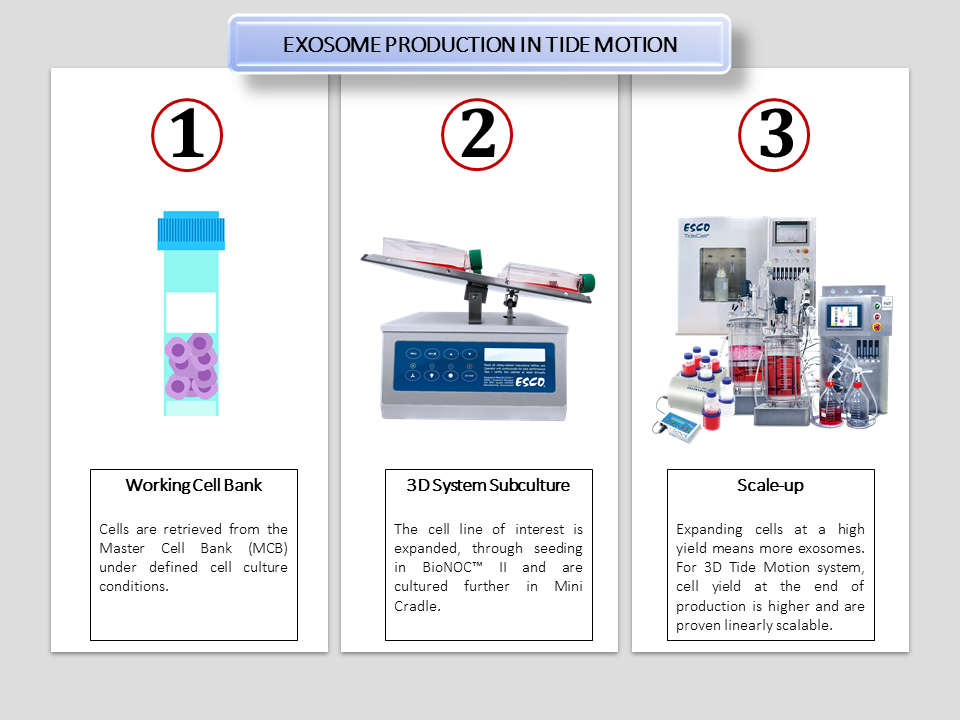
Figure 2. A working cell bank is retrieved from MCB for further production. (1) The cell line of interest is seeded in BioNOC™ II carriers and transferred in a T-flask for subculture. The T-flask with carriers is put in a 2D rocker for aeration and nutrition exchange. (2) After which the Mini Cradle culture is optimized, users can scale-up to either CelCradle™, for single-use expansion at bench-top scale; VXL™ Hybrid bioreactor, which is a versatile culture system for culturing either suspension or adherent cells; or TideCell® for option of closed system, single-use and multiple-use vessels in large-scale exosome production. (3)
Tide Motion bioreactors are linearly scalable from bench-scale to commercial scale systems. It is suitable for the scalable expansion of adherent cells and addressing upstream processing needs. The robust process defines a cost-effective attribute saving both time and effort.
The recent explosion of exosome research to produce and provide promising candidate therapies has caused global excitement. With the numerous preclinical studies showing the potency of exosomes, it is timely to address the potential technologies to address bioprocessing challenges. Understanding the biological properties of exosome cargo emerges research to underpin its potential for clinical setting.
References:
Esco VacciXcell
21 Changi South Street 1
Singapore 486777
T: +65 6542 0833
E: [email protected]
About Exosomes
Exosomes are extracellular vesicles that are released from cells upon fusion with an intermediate endocytic compartment, or a multivesicular body (MVB). They serve as a “vehicle” for transporting bioactive molecules to a target cell. Source: https://www.news-medical.net/life-sciences/What-are-Exosomes.aspx
About Tide Motion Bioreactors
Tide Motion pertains to the gentle oscillation of culture medium into and out of the matrix vessel that intermittently exposes the cells to aeration and nutrition. The upward oscillation exposes the cells to nutrition, while the downward oscillation exposes the cells to aeration. At the same time, this process washes away products and wastes. This oscillation produces no air bubbles and low shear stress. View a range of products at http://www.vaccixcell.com/tide-technology/
About Esco VacciXcell
Esco VacciXcell is the bioprocessing division of Esco Group of Companies that specializes in the marketing and manufacturing of bioprocessing equipment for cell culture.
Esco VacciXcell provides turnkey manufacturing solutions using its proprietary Tide Motion™ technology to help developing nations to be self-sufficient in the manufacturing, storing, distribution, and administration of vaccines and other biologics, thus providing a complete solution from Discovery to Delivery. For more information on VacciXcell, please visit www.vaccixcell.com
Sign up to our newsletter and receive the latest news and updates about our products!
