Vaccines have been one of the tools used to combat infectious diseases since the 18th century. Their ability to induce the production of protective antibodies against specific epitopes of disease-causing pathogen, has saved the lives of countless humans and animals for centuries.
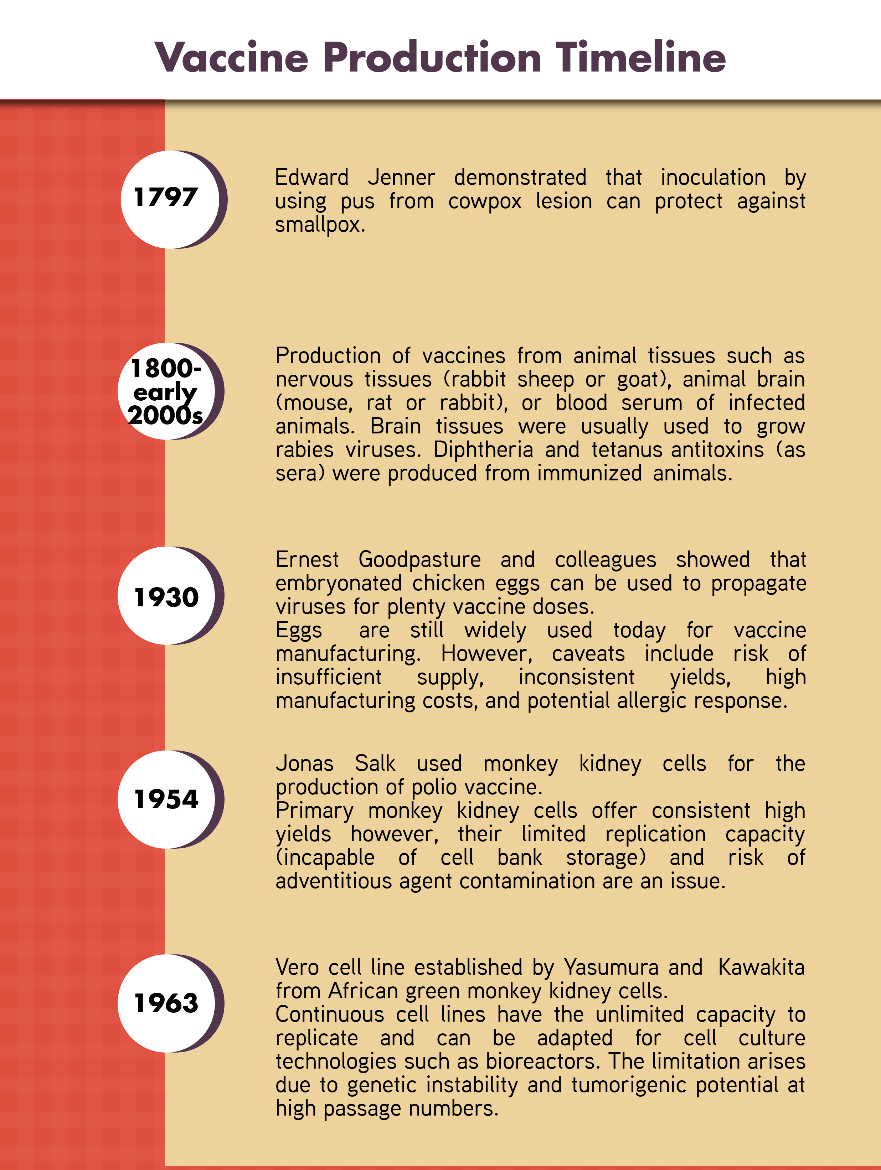
Figure 1. Timeline of the development of vaccine production technologies
Most of the well-characterized, regulatory-approved cell lines being used today for manufacturing vaccines against viral diseases were developed and stored in cell bank repositories from 1960-70s. These stored cell lines are already 40-50 years old and the stocks are continuously depleting as vaccines against hepatitis A, polio, rabies, and influenza are being mass produced daily. Regulatory bodies only allow low passage numbers are to be used to manufacture vaccines. Otherwise, high-passage number vaccines may have tumorigenic potential and can cause side effects when injected. To keep the population protected from these viruses, including emerging and future ones, there is a motivation to develop new cell lines capable of propagating broad range of viruses. Designer cells have been developed to overcome the depleting supply of traditional cell lines. These designer cell lines address the directed development of cells for specific production processes such as monoclonal vaccines, recombinant proteins, or viral vaccines. Established or new cell lines undergo genetic modification, designing for specific application, or in other studies screened for multiple applications. The cell lines for vaccine production should ideally be stable up to 100 passages, adapted to serum-free medium, propagate a broad range of viruses (universal), and produce high titers of viruses.
The appropriate host should be chosen to extract tissues needed for the establishment of new cell lines. The risk posed by endogenous retroviruses (ERV) present on animals should be considered, and thus, should undergo additional testing during production to ensure that the resulting cells are ERV-free. On the other hand, human donors are a controversial topic for tissue extraction as some designer cells originated from abortion procedures. However, the highest immune response and correct post-translational modifications are achieved using human cells. The appropriate tissue to extract is also selected based on the range of viruses and the type of immortalization method employed.
For the extracted and cultivated cells to proliferate indefinitely, immortalization is essential and can be achieved through transformation methods (e.g. SV40), exposure to chemical carcinogens, or UV irradiation. Further improvements to the newly-developed continuous cell line are made by adapting to serum-free or chemically-defined medium, bypassing rigorous regulatory requirements for testing for adventitious agents.
The developed cell lines should be screened for propagating different viruses and in high titers. Genetic modifications involving modern biotechnology tools including RNA silencing and CRISPR gene editing can be utilized to design the cells as ideal for viral propagation. Established cell lines including Vero cells can also be genetically modified for more efficient vaccine propagation. This is also intuitive as regulatory bodies would not meticulously examine a GM Vero cell line compared to a newly-developed, GM-cell line. In a study by Murray et al., RNA silencing technique is used to determine key genes involved in viral replication of poliovirus, influenza and rotavirus. In the study, silencing key genes resulted in 2-fold to over 1000 fold increase in titers. In conjunction with genome editing, permanent deletion of the key genes may result in a more permissive and efficient continuous cell line.
Once an ideal new cell line platform is developed, the master and working seeds will be frozen in properly maintained cell banks. Large stocks of the seed shall be stored for large-scale vaccine manufacturing, using enhanced bioreactors of the future. These seed stocks will be the sleeping workhorses to help us combat our viral enemies in the future.
References:
1. Aubrit, F., Perugi, F., Léon, A., Guéhenneux, F., Champion-Arnaud, P., Lahmar, M., & Schwamborn, K. (2015). Cell substrates for the production of viral vaccines. Vaccine, 33(44), 5905-5912. doi:10.1016/j.vaccine.2015.06.110.
2. Genzel, Y. (2015). Designing cell lines for viral vaccine production: Where do we stand? Biotechnology Journal, (5), 728. https://doi.org/10.1002/biot.201400388
3. Murray, J., Todd, K. V., Bakre, A., Orr-Burks, N., Jones, L., Wu, W., & Tripp, R. A. (2017). A universal mammalian vaccine cell line substrate. PLoS ONE, 12(11), 1-17. https://doi.org/10.1371/journal.pone.0188333
Sign up to our newsletter and receive the latest news and updates about our products!
