
The immune system has evolved its response to threats, protecting the host from pathogens. The ability of the immune system to differentiate itself from pathogens is through intercellular communication which is a key in this process. Cell to cell communication or signaling involves the transfer of chemokines, cytokines, and direct cell contact.
Most cells produce extracellular vesicles such as microvesicles of >200nm in size and exosomes of 50-200 nm in diameter. Microvesicles (MVs) and exosomes are shed from the plasma membrane and originate within microvesicular bodies (MVBs). Exosomes comprise a homogenous population of cup-shaped, nano-sized vesicles. Its biogenesis therefore starts at endocytosis, progresses through early and late endosomes to MVBs, and finally forms when MVBs fuse with the plasma membrane to release the intraluminal vesicles (ILVs).
Exosomes originate from almost every cell type including, but not limited to: T cells, B cells, dendritic cells, endothelial cells, and smooth muscle cells among others. The wide variety of cells that can excrete exosomes also dictates that they can be isolated from saliva, plasma, urine, cerebrospinal fluid, bronchial alveolar lavage, and even breast milk.
Exosomes are a better characterized population of extracellular vesicles. They were once thought to be “trash bins” for cells to discard unwanted proteins. However, due to the rapid changes in science, it has been found that exosomes play an essential role in intercellular communication, serving as a vehicle for mediating a wide array of cellular processes. Exosomes carry all the signaling and machinery to go and to change the behavior of a neighboring cell or distant parts of the body. This includes proteins, lipids, DNA, and perhaps the most interesting thing, messenger RNA (mRNA). mRNAs are instruction templates to make proteins, which are the basic building blocks of life.
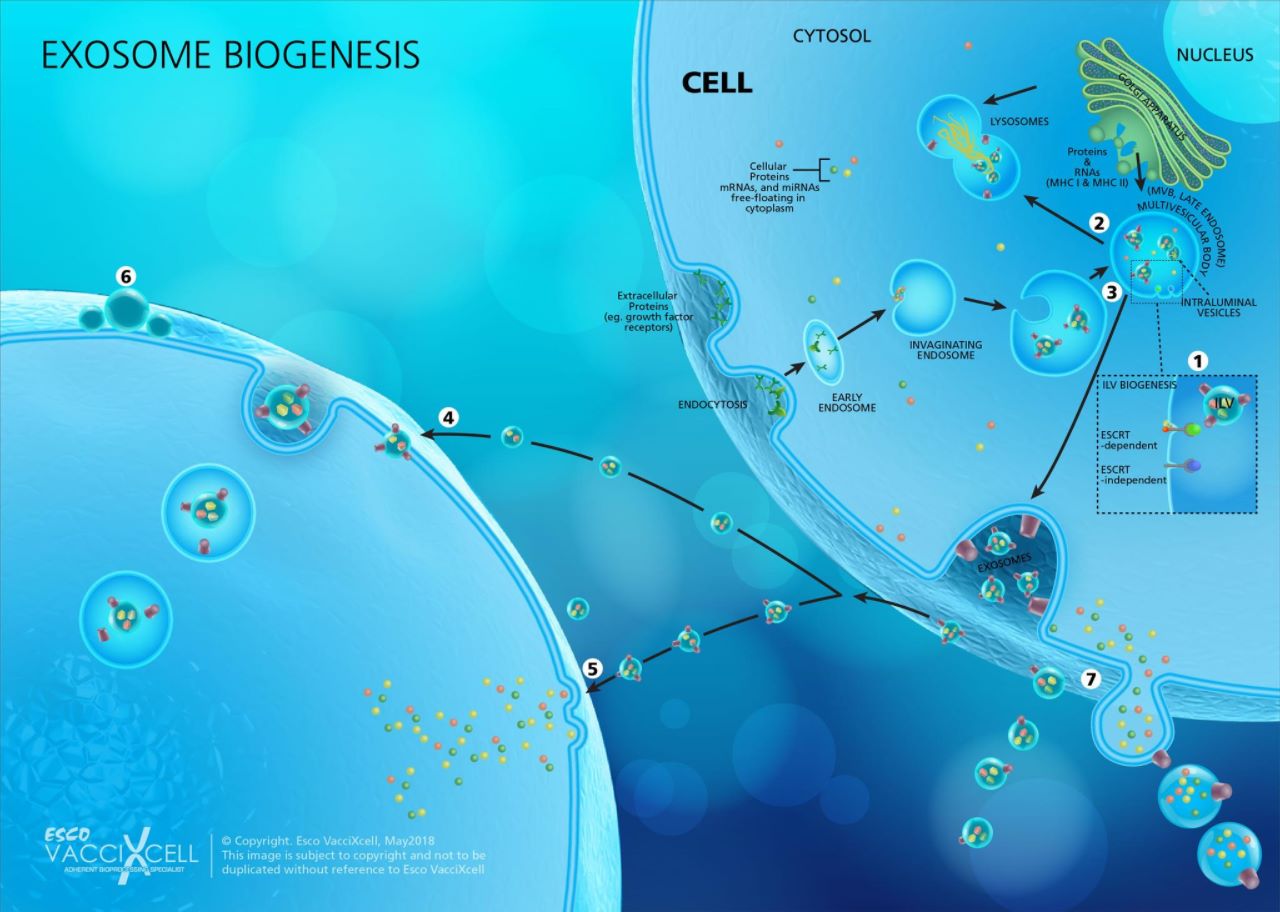
Figure 1. Exosome Biogenesis. (1) Invagination of the endosomal membrane generates Intraluminal Vesicles or ILVs in MVBs, which contain proteins, mRNAs, and miRNAs from the cytoplasm. This process can be done by either ESCRT-dependent (ESCRT stands for endosomal sorting complexes required for transport) or ESCRT-independent mechanisms. ILVs accumulated within the lumen of MVBs have three distinct fates: delivering content for the biogenesis of lysosome-related organelles; (2) fuse with lysosome, which degrade multivesicular endosomes (MVE) contents; or (3) fuse with plasma membrane where ILVs liberated into the extracellular space are released as exosomes. For exosomes to reach their destination and elicit a response from recipient cells, they may (4) be taken up by the target cell’s endocytic pathway, delivering the exosome via back-fusion event, or (5) fusing into the cell’s plasma membrane and releasing its contents directly to the cytosol. (6) Alternatively, exosomes may attach to the surface of the target cell to elicit a signaling response. (7) Membrane-derived vesicles such as ectosomes and microvesicles among others, are also secreted by the cell.
For example, virally or bacterially infected cells use exosomes as “vehicles” to spread diseases. Cancer cells utilize exosomes in metastasis and tumor progression. They have also been implicated in degenerative diseases such as Parkinson’s and Alzheimer’s, as well as for tissue regeneration. During injury, cardiomyocyte progenitor cells secrete exosomes to stimulate the migration of endothelial cells and promote cardiac regenerative activity.
Exosomes are indeed powerful mediators for both adaptive and innate immune responses, thereby participating also in antigen release in association with Major Histocompatibility Complex – II (MHC-II) and Major Histocompatibility Complex – I (MHC-I) molecules. In mice, B-lymphocyte- and dendritic cell (DC) – derived exosomes stimulate T cells and initiate host immune responses. When pulsed with tumor peptides, DC-derived exosomes prepare cytotoxic T cells and suppress tumor growth.
The nature on how exosomes work holds promise in research to utilize them for diagnostics and therapeutics. Extensive investigations on using exosomes to treat diseases such as cancer had paved its way for a novel “cell-free” therapy.
References:
About Exosomes
Exosomes are extracellular vesicles that are released from cells upon fusion with an intermediate endocytic compartment, or a multivesicular body (MVB). They serve as a “vehicle” for transporting bioactive molecules to a target cell. Source: https://www.news-medical.net/life-sciences/What-are-Exosomes.aspx
About Tide Motion Bioreactors
Tide Motion pertains to the gentle oscillation of culture medium into and out of the matrix vessel that intermittently exposes the cells to aeration and nutrition. The upward oscillation exposes the cells to nutrition, while the downward oscillation exposes the cells to aeration. At the same time, this process washes away products and wastes. This oscillation produces no air bubbles and low shear stress. View a range of products at http://www.vaccixcell.com/tide-technology/
About Esco VacciXcell
Esco VacciXcell is the bioprocessing division of Esco Group of Companies that specializes in the marketing and manufacturing of bioprocessing equipment for cell culture.
Esco VacciXcell provides turnkey manufacturing solutions using its proprietary Tide Motion™ technology to help developing nations to be self-sufficient in the manufacturing, storing, distribution, and administration of vaccines and other biologics, thus providing a complete solution from Discovery to Delivery. For more information on VacciXcell, please visit www.vaccixcell.com
Sign up to our newsletter and receive the latest news and updates about our products!
