The emerging role of exosomes as a natural nano-vehicle offers important advantages for drug delivery compared to other nanoparticulate delivery systems. Looking at a bigger picture, exosomes are a part of the family of “bioactive vesicles” that are involved in several biological and pathological processes.
Exosomes carry out intercellular communication which is essential for maintaining homeostasis and cell development in multicellular organisms. They contain transport proteins, heat shock proteins, proteins associated with mutli-vesicular bodies (MVBs), integrins, and tetraspanin within the bilayered membrane. Exosomes are also comprised of different lipids such as cholesterol, sphingolipids, phosphoglycerides, ceramides, and saturated fatty acid chains. These compositions make exosomes critical serving as biomarkers and indicators in biological processes.
The composition from which the exosome is formed is dependent from its derived parent cells. Differing exosome origins mean that they have different functions. For example, prostaglandin-carrying exosomes secreted by platelets are found to be involved in inflammatory responses. Studies have also shown that exosomes derived from antigen presenting cells can aid immune function. It expresses major histocompatibility complex (MHC)-I and II molecules on the cell surface, which helps in activating CD8 and CD4 T-cells to induce specific immune responses. CD4 cells are helper cells that lead the attack against infections while CD8 cells are suppressor cells that can kill cancer cells and other invaders.
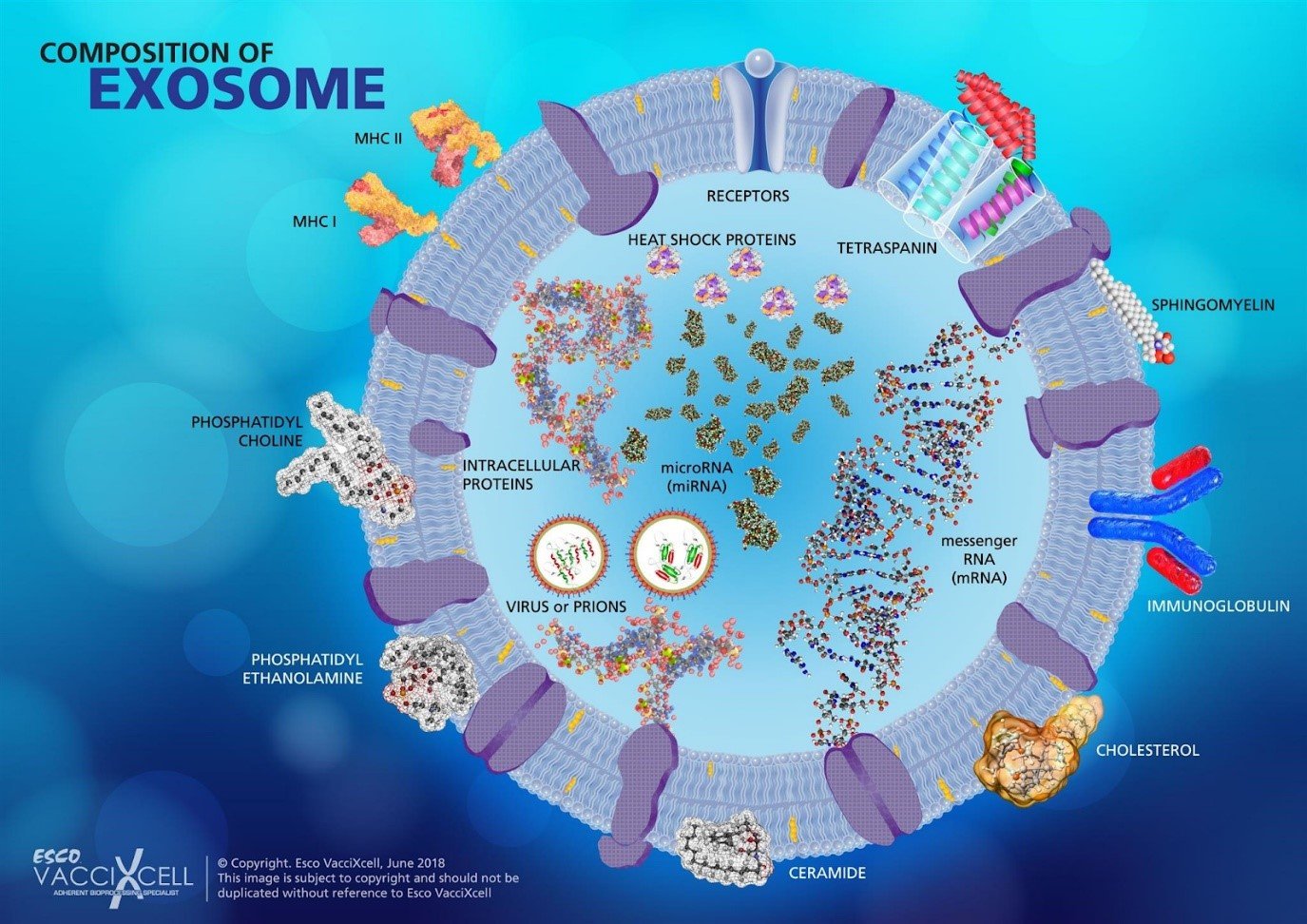
Figure 1. The composition of exosome varies from the cell of its origin.
In general, exosomes facilitate cell-to-cell communication, in a target-specific manner, throughout the body. Given that exosomes play a crucial role in the maturation of erythrocytes, antigen presentation in immune responses, coagulation, inflammation, and angiogenesis amongst others, there have been extensive studies for utilizing exosomes in both diagnostics and therapeutics.
Exosomes as drug delivery vehicle
Extensive research had utilized exosomes for delivering small molecules, proteins, and even nucleic acids for therapeutic application. For small molecules delivery, curcumin, which is naturally found in the rhizomes of turmeric, has been used to aid in treating inflammatory diseases. Antineoplastic, antioxidant, and chemo-preventive properties are enhanced when exosomes are incorporated with curcumin.
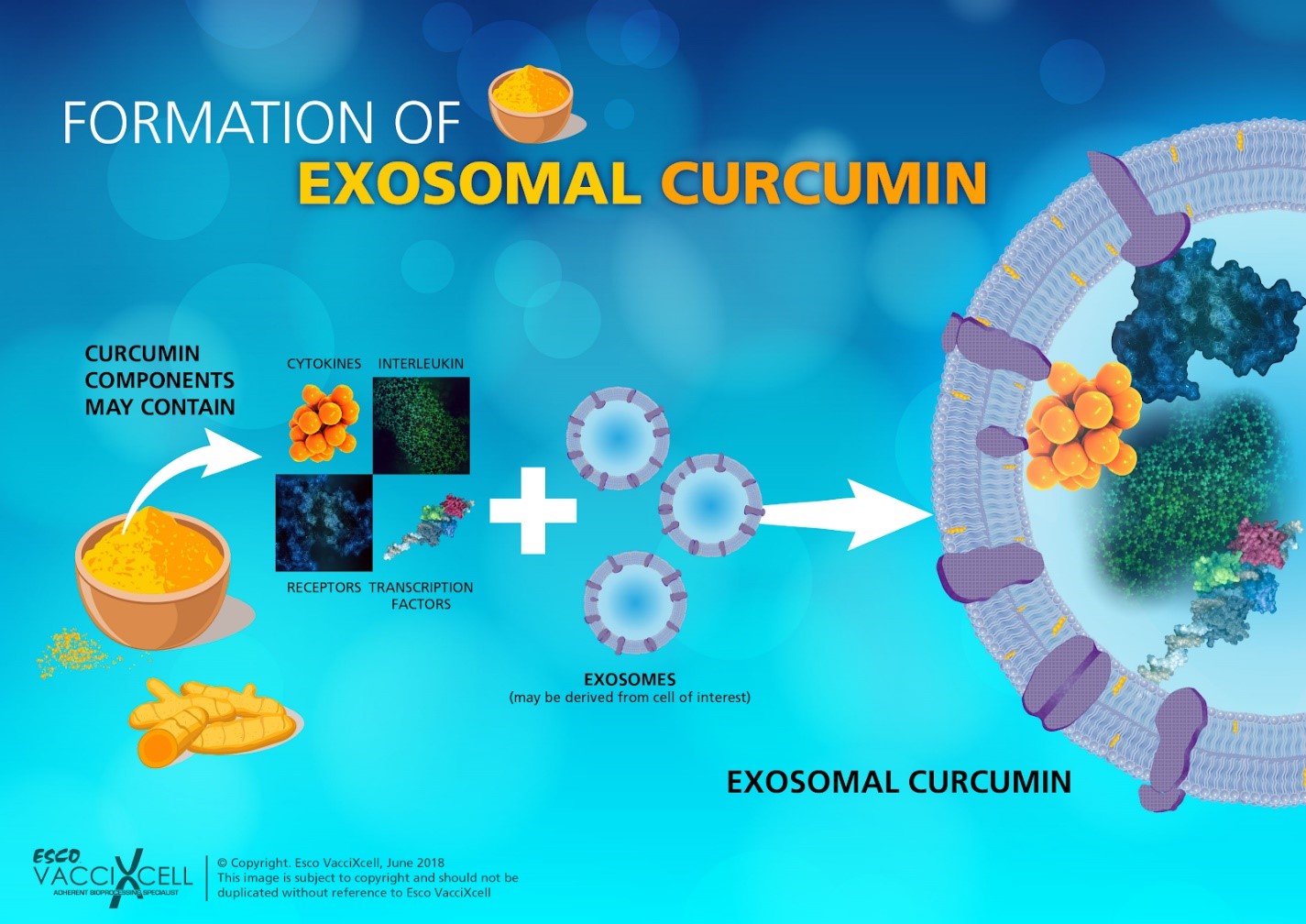
Figure 2. Curcumin contain many components that can be extracted for treating disease. Depending on the target, the components will be incorporated into an exosome derived from a chosen cell line. These exosomes will then be released in the extracellular medium after culture and isolated through centrifugation process.
Exosomes have many desirable features to become an ideal drug delivery vehicle. It has a long circulating half-life, intrinsic ability to target tissues, minimal or no inherent toxicity issues, and many more. Exosomes can carry small molecular drugs across the blood-brain barrier (BBB) in which other delivery systems cannot. A study using a zebrafish as an animal model indicated that exosomes encapsulated with anticancer drugs undeniably showed the drug’s distribution in its brain region. Since exosomes are a “natural” nano-vehicle, it can avoid phagocytosis and bypass lysosomal engulfment to completely deliver cargoes directly to the targeted cell.
Aside from delivering small molecules, exosomes are also utilized for protein delivery. One best example was indicated in a study of patients with Parkinson’s Disease (PD). This disease has lower antioxidant enzymes like catalase and superoxide dismutase. These enzymes help in oxidative stress and inhibition of neurodegeneration. Catalase is an enzyme that can deactivate one million free radicals per second per molecule in a single cycle of catalytic reaction. Catalase incorporated in exosomes targets and delivers this cargo to the brain tissue, leading to a promising option for PD therapy.
Nucleic acids such as DNA and RNA have also been shown to be naturally carried by exosomes. This feature made exosomes the major interest in gene therapy. There are treatment strategies that have utilized exosomes to deliver therapeutic genetic material that may alter the gene expression of a certain disease.
Exosomes in Immune Function
Exosomes are first found to be involved in removing unnecessary proteins during the cell maturation process. Since this research had been carried out, numerous studies had demonstrated that there is more to exosomes than just protein removal. In fact, exosomes derived from dendritic cells (DCs) can activate T-cells either by direct antigen presentation or by transferring of antigenic peptides to antigen presenting cells (APCs).
Heat shock proteins, which are molecular chaperones important for protein-protein interactions, expressed by exosomes can activate natural killer cells (NKs) and macrophages. This will help the body fight off foreign bodies through locating, controlling, and eventually killing it as a form of immune response. Moreover, it has been observed that exosomes derived from infected cells can induce a specific anti-microbial response. Toxoplasma gondii infected cells can be pulsed with DC-derived exosomes to promote anti-parasitic immunity and to confer protection in an infected individual.
Exosomes in Infectious Diseases
Infected mammalian cells or even prions secrete exosome with different purposes. Apart from the widely studied immunomodulatory effects, exosomes released by these pathogens carry virulence factors such as proteins, mRNA, and miRNA. These factors contribute to the spread of infection; therefore, the action may be beneficial or harmful to the pathogen or the host.
Depending on the virus, its life cycle, and the type of infected cell in viral infections, exosomes have been involved in various mechanisms. In the case of HIV, exosomes take part in the transfer of proteins and RNA from infected to non-infected cells. This mediated strategy aids in manipulating the host’s cell machinery. Hepatitis B, C, and E viruses also utilize the mechanism of exosomes to mediate alternative viral transmission and disease persistence. Viral RNA exported by these viruses’ exosomes could either or both serve as a viral strategy to evade the immune system (spreading the infection) and/or induce an innate response in bystander cells.
Members of the Herpes virus have also been described to utilize cellular exosomes through capturing its cellular pathway during biogenesis and release. Moreover, virus-loaded exosomes production is enhanced supporting the dissemination and persistence of viral particles. This can interfere with the transport of antigens into the host’s immune system, compromising the body’s immunological surveillance.
Exosomes secreted in response to an infection may be a novel candidate for the design of vaccines. They can also be employed as infection biomarkers as exosomes are implicated in many processes of the infection’s biology.
Exosomes in Cancer
Exosomes released in the extracellular space have multiple mechanisms to be of paramount importance in cancer disease. These mechanisms are how exosomes remodel the extracellular matrix (ECM) when released into the extracellular space, and how it promotes angiogenesis, thrombosis, and tumor cell proliferation.
Cancer progression requires direct interaction between tumor cells and its environment. Exosomes achieve this cell-cell communication through modulating nearby or distant target cells by direct contact of their surface molecules or indirect contact to activate intracellular pathways. This role of exosomes induces a pro-tumoral microenvironment for carcinogenesis and regulates the immune response to promote tumor progression and survival.
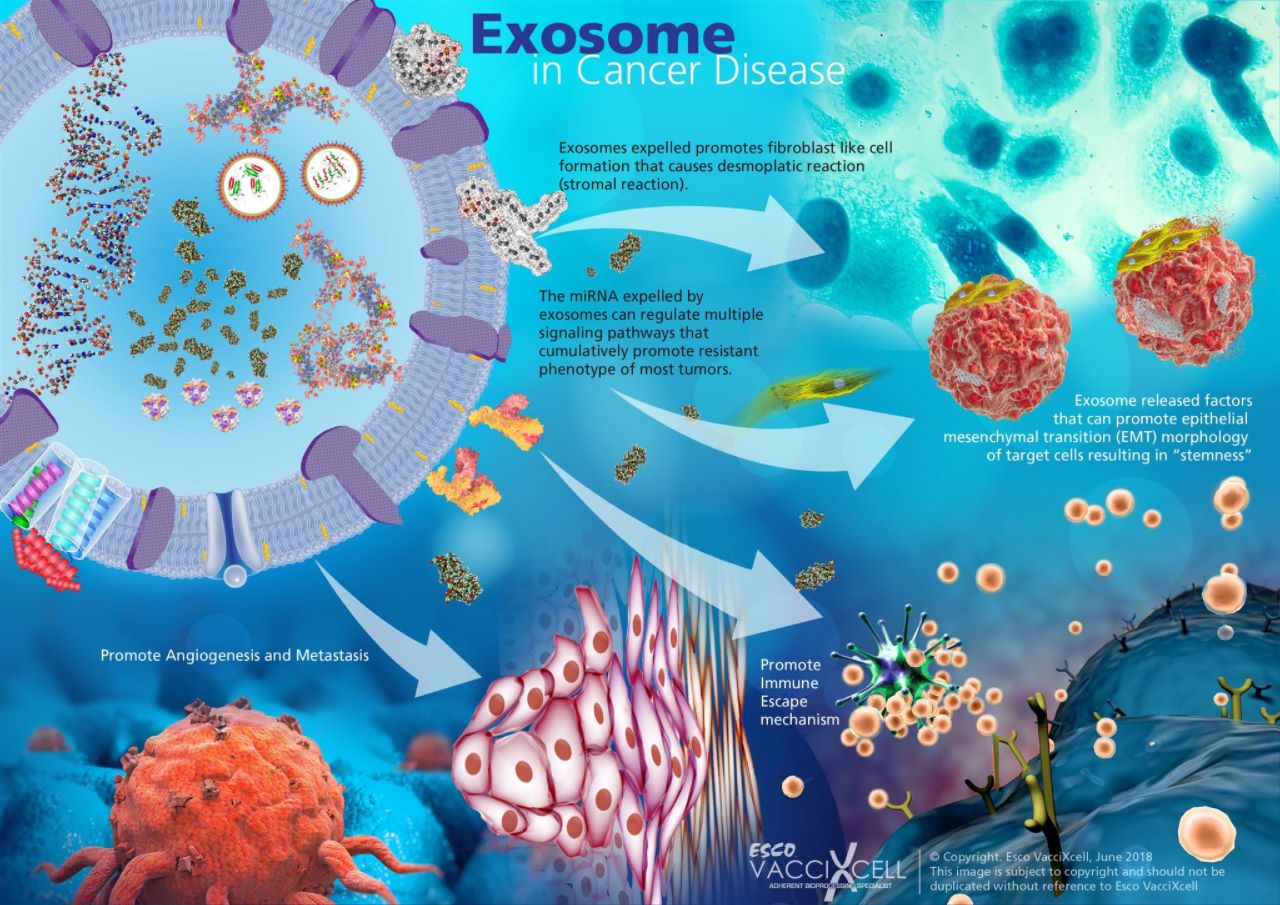
Each tumor type has preferential organs and thus before spreading (metastasis), a pre-metastatic environment forms in the target organ and prepares its tissues for metastatic cell growth and settlement. Tumor cells for example, can generate exosomes to harbor metastatic niches. This vesicle will deliver its miRNAs to communicate with neighboring endothelial cells and distant organ cells for the formation of new blood vessels (angiogenesis) and metastasis. Moreover, they can activate T cells via antigen presentation, NK cells via interaction with a specific ligand, and DCs via antigen transfer. Exosomes show remarkable inhibitory effects on cell proliferation and tumor progression and represent novel biomarkers for accurate diagnosis and prognosis of disease progression. This makes them the center of interest for an alternative cell-free vaccine for immunotherapy.
Exosomes are also associated with neurodegenerative diseases, cardiovascular diseases, and even pregnancy. These natural vesicles provide a potential clinical application for diagnosing and treating certain types of disease. Given the role and importance of exosomes in pathobiological conditions, existing and future research of exosomes will help bolster control, prevention, and therapy.
Reference:
EscoVacciXcell
21 Changi South Street 1
Singapore 486777
T: +65 6542 0833
E: [email protected]
About Exosomes
Exosomes are extracellular vesicles that are released from cells upon fusion with an intermediate endocytic compartment, or a multivesicular body (MVB). They serve as a “vehicle” for transporting bioactive molecules to a target cell. Source: https://www.news-medical.net/life-sciences/What-are-Exosomes.aspx
About Tide Motion Bioreactors
Tide Motion pertains to the gentle oscillation of culture medium into and out of the matrix vessel that intermittently exposes the cells to aeration and nutrition. The upward oscillation exposes the cells to nutrition, while the downward oscillation exposes the cells to aeration. At the same time, this process washes away products and wastes. This oscillation produces no air bubbles and low shear stress. View a range of products at http://www.vaccixcell.com/tide-technology/
About Esco VacciXcell
Esco VacciXcell is the bioprocessing division of Esco Group of Companies that specializes in the marketing and manufacturing of bioprocessing equipment for cell culture.
Esco VacciXcell provides turnkey manufacturing solutions using its proprietary Tide Motion™ technology to help developing nations to be self-sufficient in the manufacturing, storing, distribution, and administration of vaccines and other biologics, thus providing a complete solution from Discovery to Delivery. For more information on VacciXcell, please visit www.vaccixcell.com
Sign up to our newsletter and receive the latest news and updates about our products!
